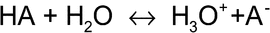1059 1059 EXCIPIENT PERFORMANCE EXCIPIENT PERFORMANCE
INTRODUCTION
Excipients are used in virtually all drug products and are essential to product performance. Thus, the successful manufacture of a robust product requires the use of well-defined excipients and processes that together yield a consistent product. Typically, excipients are manufactured and supplied to comply with compendial standards. The development, manufacture, and performance of pharmaceutical dosage forms often depend upon the physical and chemical properties of excipients that may not be provided in National Formulary (NF) monographs.
An excipient may have different functional purposes and may possess various required characteristics (e.g., particle size, particle size distribution, or surface area), depending on its use in a formulation or manufacturing process. A listing of excipients grouped by functional category is included in the NF and summarizes the most typically identified purposes these excipients serve in drug products. The list of excipients included in each category is not comprehensive and is not intended to limit in any way the choice or use of the excipient. For the complete list, refer to the USP and NF Excipients, Listed by Category in the National Formulary, under Contents.
Excipient functional category (sometimes referred to as functionality) is a broad, qualitative, and descriptive term for the purpose or role an excipient serves in a formulation. Of greater importance, however, are the quantitative performance requirements (e.g., critical material attributes) of excipients that must be evaluated and controlled to ensure consistent performance throughout the product life cycle. Not all critical material attributes of an excipient may be identified or evaluated by tests, procedures, and acceptance criteria in NF monographs. Excipient suppliers and users therefore at times may wish to identify and control critical excipient attributes that go beyond monograph specifications. This requires a thorough understanding of the formulation, the manufacturing processes, and the physical and chemical properties of each ingredient. Manufacturers should anticipate lot-to-lot and supplier-to-supplier variability in excipient properties and should have in place appropriate controls if needed to ensure consistent excipient performance.
This general chapter provides an overview of the key functional categories of excipients, tests that may assess excipient performance, and test procedures that may not be presented in compendial monographs. The functional categories have been organized by their most typical use in common pharmaceutical dosage forms (Tablets and Capsules; Oral Liquids; Semisolids, Topicals and Suppositories; Parenterals; and Aerosols) to provide a greater level of specificity for each functional category. Several functional categories (e.g., antioxidant) can apply to multiple dosage form types. The association of a functional category with a particular dosage form in this chapter is not absolute and does not limit use of an excipient to a single type of dosage form. Because of the complex nature and interplay of formulation ingredients, processing, and dosage form performance requirements, the information provided in this chapter should not be viewed as either restrictive or completely comprehensive. Each functional category includes a general description; the mechanisms by which the excipients achieve their activity; physical properties common to these excipients; chemical properties; and a list of pharmacopeial general chapters that may be useful in the development of specific tests, procedures, and acceptance criteria, and that help to ensure that the critical material attributes are adequately monitored and controlled.
TABLETS AND CAPSULES
Functional Category: Diluent
 Description:
Diluents are components that are incorporated into tablet or capsule dosage forms to increase dosage form volume or weight. Sometimes referred to as fillers, diluents often comprise a significant proportion of the dosage form, and the quantity and type of diluent selected often depend on its physical and chemical properties. Because the diluent may comprise a large portion of the dosage form, successful and robust manufacturing and dosage form performance depend on the measurement and control of the critical material attributes. Description:
Diluents are components that are incorporated into tablet or capsule dosage forms to increase dosage form volume or weight. Sometimes referred to as fillers, diluents often comprise a significant proportion of the dosage form, and the quantity and type of diluent selected often depend on its physical and chemical properties. Because the diluent may comprise a large portion of the dosage form, successful and robust manufacturing and dosage form performance depend on the measurement and control of the critical material attributes.
 Functional Mechanism:
Among the most important functional roles diluents play is their ability to impart desirable manufacturing properties (e.g., powder flow, tablet compaction strength, wet or dry granule formation, homogeneity) and performance (e.g., content uniformity, disintegration, dissolution, tablet integrity, friability, physical and chemical stability). Some diluents (e.g., microcrystalline cellulose) are occasionally referred to as dry binders because of the high degree of tablet strength they impart to the final compressed tablet. Functional Mechanism:
Among the most important functional roles diluents play is their ability to impart desirable manufacturing properties (e.g., powder flow, tablet compaction strength, wet or dry granule formation, homogeneity) and performance (e.g., content uniformity, disintegration, dissolution, tablet integrity, friability, physical and chemical stability). Some diluents (e.g., microcrystalline cellulose) are occasionally referred to as dry binders because of the high degree of tablet strength they impart to the final compressed tablet.
 Physical Properties:
The primary physical properties relevant to tablet/capsule diluents are those that can have a direct effect on diluent and formulation performance. These include: (1) particle size and size distribution, (2) particle shape, (3) bulk/tapped/true density, (4) specific surface area, (5) crystallinity, (6) moisture content, (7) powder flow, (8) solubility, and (9) compaction properties for tablet dosage forms. Physical Properties:
The primary physical properties relevant to tablet/capsule diluents are those that can have a direct effect on diluent and formulation performance. These include: (1) particle size and size distribution, (2) particle shape, (3) bulk/tapped/true density, (4) specific surface area, (5) crystallinity, (6) moisture content, (7) powder flow, (8) solubility, and (9) compaction properties for tablet dosage forms.
 Chemical Properties:
Tablet diluents comprise a large and diverse group of materials that include inorganics (e.g., dibasic calcium phosphate, calcium carbonate), single-component organic materials (e.g., lactose monohydrate, mannitol), and multicomponent or complex organics (e.g., microcrystalline cellulose, starch). They may be soluble or insoluble in water, and they may be neutral, acidic, or alkaline in nature. These chemical properties may have a positive or negative affect on the drug substance physical or chemical stability and on performance. Appropriate selection of excipients with desirable physical and chemical properties can enhance the physical and chemical stability as well as the performance of the drug substance and dosage form. The detailed composition of an excipient may be important because excipient function could be influenced by the presence of minor concomitant components that are essential for proper performance. Pharmaceutical scientists may need to control the presence of undesirable components (e.g., heavy metals or peroxides) to ensure adequate dosage form stability and performance. Chemical Properties:
Tablet diluents comprise a large and diverse group of materials that include inorganics (e.g., dibasic calcium phosphate, calcium carbonate), single-component organic materials (e.g., lactose monohydrate, mannitol), and multicomponent or complex organics (e.g., microcrystalline cellulose, starch). They may be soluble or insoluble in water, and they may be neutral, acidic, or alkaline in nature. These chemical properties may have a positive or negative affect on the drug substance physical or chemical stability and on performance. Appropriate selection of excipients with desirable physical and chemical properties can enhance the physical and chemical stability as well as the performance of the drug substance and dosage form. The detailed composition of an excipient may be important because excipient function could be influenced by the presence of minor concomitant components that are essential for proper performance. Pharmaceutical scientists may need to control the presence of undesirable components (e.g., heavy metals or peroxides) to ensure adequate dosage form stability and performance.
 General Chapters:
The following general chapters may be useful in ensuring consistency in diluent functions: Bulk Density and Tapped Density of Powders General Chapters:
The following general chapters may be useful in ensuring consistency in diluent functions: Bulk Density and Tapped Density of Powders  616 616 , Density of Solids , Density of Solids  699 699 , Crystallinity , Crystallinity  695 695 , Crystallinity Determination by Solution Calorimetry , Crystallinity Determination by Solution Calorimetry  696 696 , Loss on Drying , Loss on Drying  731 731 , Water Determination , Water Determination  921 921 , Optical Microscopy , Optical Microscopy  776 776 , Particle Size Distribution Estimation by Analytical Sieving , Particle Size Distribution Estimation by Analytical Sieving  786 786 , Light Diffraction Measurement of Particle Size , Light Diffraction Measurement of Particle Size  429 429 , Powder Fineness , Powder Fineness  811 811 , Specific Surface Area , Specific Surface Area  846 846 , and Powder Flow , and Powder Flow  1174 1174 . .
Functional Category: Binder
 Description:
Tablet/capsule binders are incorporated into formulations to facilitate the agglomeration of powder into granules during mixing with a granulating fluid such as water, hydroalcoholic mixtures, or other solvents. The binder may be either dissolved or dispersed in the granulation liquid or blended in a dry state; other components and the granulation liquid may be added separately during agitation. Following evaporation of the granulation liquid, binders typically produce dry granules that achieve the desired properties such as granule size, size distribution, shape, content, mass, and active content. Wet granulation facilitates the further processing of the granules by improving one or more of the granule properties such as flow, handling, strength, resistance to segregation, dustiness, appearance, solubility, compaction, or drug release. Description:
Tablet/capsule binders are incorporated into formulations to facilitate the agglomeration of powder into granules during mixing with a granulating fluid such as water, hydroalcoholic mixtures, or other solvents. The binder may be either dissolved or dispersed in the granulation liquid or blended in a dry state; other components and the granulation liquid may be added separately during agitation. Following evaporation of the granulation liquid, binders typically produce dry granules that achieve the desired properties such as granule size, size distribution, shape, content, mass, and active content. Wet granulation facilitates the further processing of the granules by improving one or more of the granule properties such as flow, handling, strength, resistance to segregation, dustiness, appearance, solubility, compaction, or drug release.
 Functional Mechanism:
Binders are soluble or partially soluble in the granulating solvent or, as in the case of native starches, can be made soluble. Concentrated binder solutions also have adhesive properties. Upon addition of liquid, binders typically facilitate the production of moist granules (agglomerates) by altering interparticle adhesion. They may also modify interfacial properties, viscosity, and/or other properties. During drying they may produce solid bridges that yield significant residual dry granule strength. Functional Mechanism:
Binders are soluble or partially soluble in the granulating solvent or, as in the case of native starches, can be made soluble. Concentrated binder solutions also have adhesive properties. Upon addition of liquid, binders typically facilitate the production of moist granules (agglomerates) by altering interparticle adhesion. They may also modify interfacial properties, viscosity, and/or other properties. During drying they may produce solid bridges that yield significant residual dry granule strength.
 Physical Properties:
Dispersion or dissolution of a binder in the granulation liquid depends on its physical properties: surface tension, particle size, size distribution, solubility, and viscosity are among the important properties depending on the application. Homogeneous incorporation of binder into a dry blend also depends on its physical properties such as particle size, shape, and size distribution. Viscosity often is an important property to consider for binders and, for polymers, is influenced by the nature of the polymer structure, molecular weight, and molecular weight distribution. Polymeric binders may form gels. Physical Properties:
Dispersion or dissolution of a binder in the granulation liquid depends on its physical properties: surface tension, particle size, size distribution, solubility, and viscosity are among the important properties depending on the application. Homogeneous incorporation of binder into a dry blend also depends on its physical properties such as particle size, shape, and size distribution. Viscosity often is an important property to consider for binders and, for polymers, is influenced by the nature of the polymer structure, molecular weight, and molecular weight distribution. Polymeric binders may form gels.
 Chemical Properties:
Tablet/capsule binders may be categorized as (1) natural polymers, (2) synthetic polymers, or (3) sugars. The chemical nature of polymers, including polymeric structure, monomer properties and sequence, functional groups, degree of substitution, and cross-linking influence the complex interactions that can occur during granulation. Natural polymers in particular may exhibit greater variation in their properties because of variations in their sources and therefore their composition. Chemical Properties:
Tablet/capsule binders may be categorized as (1) natural polymers, (2) synthetic polymers, or (3) sugars. The chemical nature of polymers, including polymeric structure, monomer properties and sequence, functional groups, degree of substitution, and cross-linking influence the complex interactions that can occur during granulation. Natural polymers in particular may exhibit greater variation in their properties because of variations in their sources and therefore their composition.
Functional Category: Disintegrant
 Description:
Disintegrants are functional components that are added to formulations to promote rapid disintegration into smaller units and to allow a drug substance to dissolve more rapidly. Disintegrants are natural, synthetic, or chemically modified natural polymeric substances. When disintegrants come in contact with water or stomach or intestinal fluid they function by absorbing liquid and start to swell, dissolve, or form gels. This causes the tablet structure to rupture and disintegrate, producing increased surfaces for enhanced dissolution of the drug substance. Description:
Disintegrants are functional components that are added to formulations to promote rapid disintegration into smaller units and to allow a drug substance to dissolve more rapidly. Disintegrants are natural, synthetic, or chemically modified natural polymeric substances. When disintegrants come in contact with water or stomach or intestinal fluid they function by absorbing liquid and start to swell, dissolve, or form gels. This causes the tablet structure to rupture and disintegrate, producing increased surfaces for enhanced dissolution of the drug substance.
 Functional Mechanism(s):
The ability to interact strongly with water is essential to disintegrant function. Four major mechanisms describe the function of the various disintegrants: volume increase by swelling, deformation, capillary action (wicking), and repulsion. In tablet formulations, the function of disintegrants is best described as a combination of two or more of these effects. The onset and degree of the locally achieved actions depend on various parameters of a disintegrant, such as its chemical nature and its particle size distribution and particle shape, as well as some important tablet parameters such as hardness and porosity. Functional Mechanism(s):
The ability to interact strongly with water is essential to disintegrant function. Four major mechanisms describe the function of the various disintegrants: volume increase by swelling, deformation, capillary action (wicking), and repulsion. In tablet formulations, the function of disintegrants is best described as a combination of two or more of these effects. The onset and degree of the locally achieved actions depend on various parameters of a disintegrant, such as its chemical nature and its particle size distribution and particle shape, as well as some important tablet parameters such as hardness and porosity.
 Physical Properties:
The primary physical properties relevant to a disintegrant are those that describe the product's particle structure as a dry powder or its structure when in contact with water. These properties include (1) particle size distribution, (2) water absorption rate, (3) swelling ratio or swelling index, and (4) the characterization of the resulting product whether it is still particulate or a gel is formed. Physical Properties:
The primary physical properties relevant to a disintegrant are those that describe the product's particle structure as a dry powder or its structure when in contact with water. These properties include (1) particle size distribution, (2) water absorption rate, (3) swelling ratio or swelling index, and (4) the characterization of the resulting product whether it is still particulate or a gel is formed.
 Chemical Properties:
Polymers used as disintegrants are either nonionic or anionic with counterions such as sodium, calcium, or potassium. Nonionic polymers are natural or physically modified polysaccharides such as starches, celluloses, pullulan, or cross-linked polyvinylpyrrolidone. The anionic polymers mainly are chemically modified cellulose products or low-crosslinked polyacrylates. These chemical properties should be considered in the case of ionic polymers. Disintegration performance will be affected by pH changes in the gastrointestinal tract or by complex formation with ionic active pharmaceutical ingredients (APIs). Chemical Properties:
Polymers used as disintegrants are either nonionic or anionic with counterions such as sodium, calcium, or potassium. Nonionic polymers are natural or physically modified polysaccharides such as starches, celluloses, pullulan, or cross-linked polyvinylpyrrolidone. The anionic polymers mainly are chemically modified cellulose products or low-crosslinked polyacrylates. These chemical properties should be considered in the case of ionic polymers. Disintegration performance will be affected by pH changes in the gastrointestinal tract or by complex formation with ionic active pharmaceutical ingredients (APIs).
Functional Category: Lubricant
 Description:
Lubricants typically are used to reduce the frictional forces between particles, and between particles and metal contact surfaces of manufacturing equipment such as tablet punches and dies used in the manufacture of solid dosage forms. Liquid lubricants may be absorbed into the granule matrix before compaction. Liquid lubricants also may be used to reduce metal–metal friction on manufacturing equipment. Description:
Lubricants typically are used to reduce the frictional forces between particles, and between particles and metal contact surfaces of manufacturing equipment such as tablet punches and dies used in the manufacture of solid dosage forms. Liquid lubricants may be absorbed into the granule matrix before compaction. Liquid lubricants also may be used to reduce metal–metal friction on manufacturing equipment.
 Functional Mechanism:
Boundary lubricants function by adhering to solid surfaces (granules and machine parts) and reducing the particle–particle friction or the particle–metal friction. The orientation of the adherent lubricant particles is influenced by the properties of the substrate surface. For optimal performance, the boundary lubricant particles should be composed of small, plate-like crystals or stacks of plate-like crystals. Fluid film lubricants melt under pressure and thereby create a thin fluid film around particles and on the surface of punches and dies in tablet presses, which helps to reduce friction. Fluid film lubricants resolidify after the pressure is removed. Liquid lubricants are released from the granules under pressure and create a fluid film. They do not resolidify when the pressure is removed but are reabsorbed or redistributed through the tablet matrix over the course of time. Functional Mechanism:
Boundary lubricants function by adhering to solid surfaces (granules and machine parts) and reducing the particle–particle friction or the particle–metal friction. The orientation of the adherent lubricant particles is influenced by the properties of the substrate surface. For optimal performance, the boundary lubricant particles should be composed of small, plate-like crystals or stacks of plate-like crystals. Fluid film lubricants melt under pressure and thereby create a thin fluid film around particles and on the surface of punches and dies in tablet presses, which helps to reduce friction. Fluid film lubricants resolidify after the pressure is removed. Liquid lubricants are released from the granules under pressure and create a fluid film. They do not resolidify when the pressure is removed but are reabsorbed or redistributed through the tablet matrix over the course of time.
 Physical Properties:
The primary physical properties that may be important for the function of boundary lubricants include particle size, surface area, hydration state, and polymorphic form. Purity (e.g., stearate:palmitate ratio) and moisture content also may be important. The primary physical properties of possible importance for fluid film lubricants are particle size and solid state/thermal behavior. Purity may also be important. Physical Properties:
The primary physical properties that may be important for the function of boundary lubricants include particle size, surface area, hydration state, and polymorphic form. Purity (e.g., stearate:palmitate ratio) and moisture content also may be important. The primary physical properties of possible importance for fluid film lubricants are particle size and solid state/thermal behavior. Purity may also be important.
 Chemical Properties:
Lubricants can be classified as boundary lubricants, fluid film lubricants, or liquid lubricants. Boundary lubricants are salts of long-chain fatty acids (e.g., magnesium stearate) or fatty acid esters (e.g., sodium stearyl fumarate) with a polar head and fatty acid tail. Fluid film lubricants are solid fats (e.g., hydrogenated vegetable oil, type 1), glycerides (glyceryl behenate and distearate), or fatty acids (e.g., stearic acid) that melt when subjected to pressure. Liquid lubricants are liquid materials that are released from granules under pressure. Chemical Properties:
Lubricants can be classified as boundary lubricants, fluid film lubricants, or liquid lubricants. Boundary lubricants are salts of long-chain fatty acids (e.g., magnesium stearate) or fatty acid esters (e.g., sodium stearyl fumarate) with a polar head and fatty acid tail. Fluid film lubricants are solid fats (e.g., hydrogenated vegetable oil, type 1), glycerides (glyceryl behenate and distearate), or fatty acids (e.g., stearic acid) that melt when subjected to pressure. Liquid lubricants are liquid materials that are released from granules under pressure.
 Other Information:
Certain lubricants, particularly those used in effervescent dosage forms, do not fall into the chemical categories defined above. These materials are used in special situations, and they are not suitable for universal application. Talc is an inorganic material that may have some lubricant properties. It is generally used in combination with fluid film lubricants to reduce sticking to punches and dies. Other Information:
Certain lubricants, particularly those used in effervescent dosage forms, do not fall into the chemical categories defined above. These materials are used in special situations, and they are not suitable for universal application. Talc is an inorganic material that may have some lubricant properties. It is generally used in combination with fluid film lubricants to reduce sticking to punches and dies.
Functional Category: Coloring Agent
 Description:
Coloring agents are incorporated into dosage forms in order to produce a distinctive appearance that may serve to differentiate a particular formulation from others that have a similar physical appearance. These substances are subdivided into dyes (water-soluble substances), lakes (insoluble forms of a dye that result from its irreversible adsorption onto a hydrous metal oxide), inorganic pigments (substances such as titanium dioxide or iron oxides), and natural colorants (colored compounds not considered dyes per se, such as riboflavin). Coloring agents are subject to federal regulations, and consequently the current regulatory status of a given substance must be determined before its use. Description:
Coloring agents are incorporated into dosage forms in order to produce a distinctive appearance that may serve to differentiate a particular formulation from others that have a similar physical appearance. These substances are subdivided into dyes (water-soluble substances), lakes (insoluble forms of a dye that result from its irreversible adsorption onto a hydrous metal oxide), inorganic pigments (substances such as titanium dioxide or iron oxides), and natural colorants (colored compounds not considered dyes per se, such as riboflavin). Coloring agents are subject to federal regulations, and consequently the current regulatory status of a given substance must be determined before its use.
The Federal Food, Drug, and Cosmetic Act defines three categories of coloring agents:
-
FD&C colors: those certifiable for use in coloring foods, drugs, and cosmetics
-
D&C colors: dyes and pigments considered safe in drugs and cosmetics when in contact with mucous membranes or when ingested
-
Ext. D&C colors: colorants that, because of their oral toxicity, are not certifiable for use in ingestible products but are considered safe for use in externally applied products.
 Functional Mechanism:
Water-soluble dyes usually are dissolved in a granulating fluid for use, although they may also be adsorbed onto carriers such as starch, lactose, or sugar from aqueous or alcoholic solutions. These latter products are often dried and used as formulation ingredients. Because of their insoluble character, lakes are almost always blended with other dry excipients during formulation. For this reason, direct-compression tablets are often colored with lakes. Functional Mechanism:
Water-soluble dyes usually are dissolved in a granulating fluid for use, although they may also be adsorbed onto carriers such as starch, lactose, or sugar from aqueous or alcoholic solutions. These latter products are often dried and used as formulation ingredients. Because of their insoluble character, lakes are almost always blended with other dry excipients during formulation. For this reason, direct-compression tablets are often colored with lakes.
 Physical Properties:
Particle size and size distribution of dyes and lakes can influence product processing times (blending and dissolution), color intensity, and uniformity of appearance. Physical Properties:
Particle size and size distribution of dyes and lakes can influence product processing times (blending and dissolution), color intensity, and uniformity of appearance.
 Chemical Properties:
The most important properties of a coloring agent are its depth of color and resistance to fading over time. Substances can be graded on their efficiency in reflecting desired colors of visible light, as well as on their molar absorptivities at characteristic wavelengths. A coloring agent should be physically and chemically nonreactive with other excipients and the drug substances. The quality of a coloring agent ordinarily is measured by a determination of its strength, performance, or assay. The impurity profile is established by measurements of insoluble matter, inorganic salt content, metal content, and organic impurities. Chemical Properties:
The most important properties of a coloring agent are its depth of color and resistance to fading over time. Substances can be graded on their efficiency in reflecting desired colors of visible light, as well as on their molar absorptivities at characteristic wavelengths. A coloring agent should be physically and chemically nonreactive with other excipients and the drug substances. The quality of a coloring agent ordinarily is measured by a determination of its strength, performance, or assay. The impurity profile is established by measurements of insoluble matter, inorganic salt content, metal content, and organic impurities.
 Other Information:
Coloring agents are subject to federal regulations, and consequently the current regulatory status of a given substance must be determined before it is used. Following is a list of coloring agents and currently applicable sections of the Code of Federal Regulations (CFR). Other Information:
Coloring agents are subject to federal regulations, and consequently the current regulatory status of a given substance must be determined before it is used. Following is a list of coloring agents and currently applicable sections of the Code of Federal Regulations (CFR).
| Color |
CFR |
| Ferric Oxides |
21 CFR 73.1200 |
| Titanium Dioxide |
21 CFR 73.575 & 21 CFR 73.1575 |
| FD&C Blue #1/Brilliant Blue FCF Aluminum Lake |
21 CFR 82.51 & 21 CFR 82.101 |
| FD&C Blue #2/Indigo Carmine Aluminum Lake |
21 CFR 82.51 & 21 CFR 82.102 |
| FD&C Red #40/Allura Red AC Aluminum Lake |
21 CFR 74.340 & 21 CFR 74.1340 |
| FD&C Yellow #5/Tartrazine Aluminum Lake |
21 CFR 82.51 & 21 CFR 82.705 |
| FD&C Yellow #6/Sunset Yellow FCF Aluminum Lake |
21 CFR 82.51 & 21 CFR 82.706 |
| D&C Yellow #10 Aluminum Lake |
21 CFR 82.1051 & 21 CFR 82.1710 |
| D&C Red #30/Helendon Pink Aluminum Lake |
21 CFR 82.1051 & 21 CFR 82.1330 |
| D&C Red #7/Lithol Rubin B Calcium Lake |
21 CFR 82.1051 & 21 CFR 82.1307 |
| D&C Red #27/Phloxine Aluminum Lake |
21 CFR 82.1051 & 21 CFR 82.1327 |
Functional Category: Capsule Shell
 Description:
The word capsule is derived from the Latin capsula, which means a small container. Among other benefits, capsules enable pharmaceutical powders and liquids to be formulated for dosing accuracy as well as ease of transportation. The capsule material should be compatible with all other ingredients in the drug product. Hard capsules typically consist of two parts: both are cylindrical, and one part is slightly longer than the other and is called the body. The cap fits closely on the body to enclose the capsule. In contrast, the soft capsule is a one-piece unit that may be seamed along an axis or may be seamless. The capsule material may be derived from hydrolysis of collagen that originates from porcine, bovine, or fish sources, or it can be of non-animal origin, e.g., cellulosic or polysaccharide chemical entities. The capsule shell also contains other additives such as plasticizers, colorants, and preservatives. In some cases, capsule shells are sterilized to prevent microbial growth. The capsule shell is an integral part of the formulation, and therefore robust manufacturing and formulation performance depends on the measurement and control of critical attributes. Description:
The word capsule is derived from the Latin capsula, which means a small container. Among other benefits, capsules enable pharmaceutical powders and liquids to be formulated for dosing accuracy as well as ease of transportation. The capsule material should be compatible with all other ingredients in the drug product. Hard capsules typically consist of two parts: both are cylindrical, and one part is slightly longer than the other and is called the body. The cap fits closely on the body to enclose the capsule. In contrast, the soft capsule is a one-piece unit that may be seamed along an axis or may be seamless. The capsule material may be derived from hydrolysis of collagen that originates from porcine, bovine, or fish sources, or it can be of non-animal origin, e.g., cellulosic or polysaccharide chemical entities. The capsule shell also contains other additives such as plasticizers, colorants, and preservatives. In some cases, capsule shells are sterilized to prevent microbial growth. The capsule shell is an integral part of the formulation, and therefore robust manufacturing and formulation performance depends on the measurement and control of critical attributes.
 Functional Mechanism:
Capsules can enclose solid as well as semisolid and liquid formulations. Capsules have a variety of benefits including the following: masking unpleasant taste, facilitating blinding in clinical studies, promoting ease of swallowing, and presenting a unique appearance. Conventional capsule shells should dissolve rapidly at 37 Functional Mechanism:
Capsules can enclose solid as well as semisolid and liquid formulations. Capsules have a variety of benefits including the following: masking unpleasant taste, facilitating blinding in clinical studies, promoting ease of swallowing, and presenting a unique appearance. Conventional capsule shells should dissolve rapidly at 37 in biological fluids such as gastric and intestinal media. However, the solubility properties of the shell can be modified, e.g., with enteric and controlled-release polymers, to control the release of capsule contents. in biological fluids such as gastric and intestinal media. However, the solubility properties of the shell can be modified, e.g., with enteric and controlled-release polymers, to control the release of capsule contents.
 Physical Properties:
The primary physical properties relevant to the capsule shell are those that can have a direct effect on product performance: (1) moisture content, (2) gas permeability, (3) stability on storage, (4) disintegration, (5) compactness, and (6) brittleness. The moisture content varies with the type of capsule. Hard gelatin capsules typically contain 13%–16% water compared to hypromellose (hydroxypropyl methylcellulose/HPMC) capsules that typically contain 4%–7% water content. Soft gelatin capsules contain 6%–8% water. Moisture content has a significant impact on capsule brittleness. Equilibrium water content also may be crucial to dosage form stability because water migration will take place between the shell and capsule contents. Gas permeability may be important and generally is greater for HPMC capsules than gelatin capsules because of the presence of open structures. Gelatin capsules may undergo cross-linking upon storage at elevated temperature and humidity (e.g. 40 Physical Properties:
The primary physical properties relevant to the capsule shell are those that can have a direct effect on product performance: (1) moisture content, (2) gas permeability, (3) stability on storage, (4) disintegration, (5) compactness, and (6) brittleness. The moisture content varies with the type of capsule. Hard gelatin capsules typically contain 13%–16% water compared to hypromellose (hydroxypropyl methylcellulose/HPMC) capsules that typically contain 4%–7% water content. Soft gelatin capsules contain 6%–8% water. Moisture content has a significant impact on capsule brittleness. Equilibrium water content also may be crucial to dosage form stability because water migration will take place between the shell and capsule contents. Gas permeability may be important and generally is greater for HPMC capsules than gelatin capsules because of the presence of open structures. Gelatin capsules may undergo cross-linking upon storage at elevated temperature and humidity (e.g. 40 /75% RH), but under these conditions HPMC capsules do not cross-link. The aldehyde content in the powder fill should be considered because it can promote cross-linking of gelatin shell material. Gelatin capsules should disintegrate within 15 minutes when exposed to 0.5% hydrochloric acid at 36 /75% RH), but under these conditions HPMC capsules do not cross-link. The aldehyde content in the powder fill should be considered because it can promote cross-linking of gelatin shell material. Gelatin capsules should disintegrate within 15 minutes when exposed to 0.5% hydrochloric acid at 36 –38 –38 but not below 30 but not below 30 . HPMC capsules also can disintegrate below 30 . HPMC capsules also can disintegrate below 30 . .
 Chemical Properties:
Gelatin is a commercial protein derived from native protein collagen. The product is obtained by partial hydrolysis of collagen derived from skin, white connective tissue, and bones of animals. Type A gelatin is derived by acid treatment, and Type B gelatin is derived from base treatment. The common sources of commercial gelatin are pigskin, cattle hide, cattle bone, cod skin, and tilapia skin. The gelatin capsule shell also typically contains coloring agents, plasticizers such as polyhydric alcohols, natural gums and sugars, and preservatives such as sodium metabisulfite and esters of p-hydroxybenzoic acid. The more commonly used nongelatin capsules today are made from HPMC. Different capsule types contain different moisture levels and may thus influence drug product stability. The detailed composition of an excipient may be important because the shell function can be influenced by small amounts of impurities in the excipients (e.g., peroxides in oils or aldehydes in lactose and starches) that can cause capsule cross-linking. The presence in capsule shells of undesirable materials such as metals, odorants, water-insoluble substances, and sulfur dioxide should be evaluated to ensure stability and performance. Chemical Properties:
Gelatin is a commercial protein derived from native protein collagen. The product is obtained by partial hydrolysis of collagen derived from skin, white connective tissue, and bones of animals. Type A gelatin is derived by acid treatment, and Type B gelatin is derived from base treatment. The common sources of commercial gelatin are pigskin, cattle hide, cattle bone, cod skin, and tilapia skin. The gelatin capsule shell also typically contains coloring agents, plasticizers such as polyhydric alcohols, natural gums and sugars, and preservatives such as sodium metabisulfite and esters of p-hydroxybenzoic acid. The more commonly used nongelatin capsules today are made from HPMC. Different capsule types contain different moisture levels and may thus influence drug product stability. The detailed composition of an excipient may be important because the shell function can be influenced by small amounts of impurities in the excipients (e.g., peroxides in oils or aldehydes in lactose and starches) that can cause capsule cross-linking. The presence in capsule shells of undesirable materials such as metals, odorants, water-insoluble substances, and sulfur dioxide should be evaluated to ensure stability and performance.
Functional Category: Coating Agent
 Description:
Reasons for coating pharmaceutical dosage forms include masking unpleasant tastes or odors, improving ingestion and appearance, protecting active ingredients from the environment, and modifying the release of the active ingredient (e.g., controlled-release rates or gastrointestinal targeting). The materials used as coating agents include natural, semisynthetic, and synthetic materials. These may be powders or colloidal dispersions (latexes or pseudolatexes) that usually are applied as solutions or dispersions in aqueous or nonaqueous systems. Waxes and lipids may be applied as coatings in the molten state without the use of solvents. Description:
Reasons for coating pharmaceutical dosage forms include masking unpleasant tastes or odors, improving ingestion and appearance, protecting active ingredients from the environment, and modifying the release of the active ingredient (e.g., controlled-release rates or gastrointestinal targeting). The materials used as coating agents include natural, semisynthetic, and synthetic materials. These may be powders or colloidal dispersions (latexes or pseudolatexes) that usually are applied as solutions or dispersions in aqueous or nonaqueous systems. Waxes and lipids may be applied as coatings in the molten state without the use of solvents.
 Functional Mechanism:
Coating agents are composed of film-forming materials that impart desirable pharmaceutical properties such as appearance, patient acceptance, and ease of swallowing. Coating agents also may serve other functional purposes such as providing a barrier against undesirable chemical reactions or untimely release of a drug from its components. After intake, the coating may dissolve by processes such as hydration, solubilization, or disintegration, depending on the nature of the material used. Enteric coatings are insoluble in acidic (low pH) media but dissolve readily in neutral pH conditions. However, most common coatings do not have pH-specific solubility. The coating thickness may vary by application and the nature of the coating agents. In the coating process, the polymer chains spread out on the core surface and coalesce into a continuous film as the solvent evaporates. Plastic polymers, waxes, and lipid-based coatings may be applied without solvents by melting and atomization. Molten fluid droplets, upon impact on the surface of the fluidized drug particles, spread and resolidify to form film layers. Therefore, coating materials generally have the ability to form a complete and stable film around the substrate. The coating preparation typically is applied uniformly and is carefully dried to ensure that a consistent product is produced. Suitable plasticizers may be required to lower the minimum film-forming temperature of the polymer, and their potential effect on drug release should be considered. Functional Mechanism:
Coating agents are composed of film-forming materials that impart desirable pharmaceutical properties such as appearance, patient acceptance, and ease of swallowing. Coating agents also may serve other functional purposes such as providing a barrier against undesirable chemical reactions or untimely release of a drug from its components. After intake, the coating may dissolve by processes such as hydration, solubilization, or disintegration, depending on the nature of the material used. Enteric coatings are insoluble in acidic (low pH) media but dissolve readily in neutral pH conditions. However, most common coatings do not have pH-specific solubility. The coating thickness may vary by application and the nature of the coating agents. In the coating process, the polymer chains spread out on the core surface and coalesce into a continuous film as the solvent evaporates. Plastic polymers, waxes, and lipid-based coatings may be applied without solvents by melting and atomization. Molten fluid droplets, upon impact on the surface of the fluidized drug particles, spread and resolidify to form film layers. Therefore, coating materials generally have the ability to form a complete and stable film around the substrate. The coating preparation typically is applied uniformly and is carefully dried to ensure that a consistent product is produced. Suitable plasticizers may be required to lower the minimum film-forming temperature of the polymer, and their potential effect on drug release should be considered.
 Physical Properties:
Film coating is a complex process, and the characteristics of a film-forming polymer play an important role: the particle size of colloidal dispersions varies with their origin (latex, pseudolatex, or redispersed powder) and may have an effect on the film-forming mechanism. Polymer solutions or dispersions with a low viscosity and high pigment-binding capacity reduce the coating time and facilitate relatively simple and cost-effective manufacturing. The concentration–viscosity relationship for the film-forming agent should be evaluated for process optimization. The surface tension of coating preparations can influence the spray pattern in the manufacturing process. The applied coating must withstand mechanical stress during coating or packaging operations. Therefore, the film should possess high elasticity and sufficient mechanical strength. It can be useful to analyze tensile properties of isolated films. For coatings that are applied in a molten state without solvents (plastic polymers, waxes, and lipid-based coatings), melting range and melt viscosity are the properties of prime consideration. Physical Properties:
Film coating is a complex process, and the characteristics of a film-forming polymer play an important role: the particle size of colloidal dispersions varies with their origin (latex, pseudolatex, or redispersed powder) and may have an effect on the film-forming mechanism. Polymer solutions or dispersions with a low viscosity and high pigment-binding capacity reduce the coating time and facilitate relatively simple and cost-effective manufacturing. The concentration–viscosity relationship for the film-forming agent should be evaluated for process optimization. The surface tension of coating preparations can influence the spray pattern in the manufacturing process. The applied coating must withstand mechanical stress during coating or packaging operations. Therefore, the film should possess high elasticity and sufficient mechanical strength. It can be useful to analyze tensile properties of isolated films. For coatings that are applied in a molten state without solvents (plastic polymers, waxes, and lipid-based coatings), melting range and melt viscosity are the properties of prime consideration.
 Chemical Properties:
Film-forming agents are of natural, semisynthetic, or synthetic origin and are available in different chemical grades. NF monographs often describe classes of polymeric materials that allow a considerable range of composition, structure, or molecular weight. These factors should be considered when pharmaceutical scientists identify and quantitate critical material attributes to ensure consistent performance. Chemical Properties:
Film-forming agents are of natural, semisynthetic, or synthetic origin and are available in different chemical grades. NF monographs often describe classes of polymeric materials that allow a considerable range of composition, structure, or molecular weight. These factors should be considered when pharmaceutical scientists identify and quantitate critical material attributes to ensure consistent performance.
 Additional Information:
Additives often are included in a coating formulation. Fillers (e.g., sugar alcohols, microcrystalline cellulose) may be added to increase the solids content of the coating agent without increasing viscosity. Stearic acid can be used to improve the protective function/moisture barrier of a coating. Water-soluble or -insoluble ingredients may be added to create pores in the film to adjust the release pattern of sustained-release formulations. Coloring agents (e.g., titanium dioxide, kaolin) may be added to modify appearance. Additional Information:
Additives often are included in a coating formulation. Fillers (e.g., sugar alcohols, microcrystalline cellulose) may be added to increase the solids content of the coating agent without increasing viscosity. Stearic acid can be used to improve the protective function/moisture barrier of a coating. Water-soluble or -insoluble ingredients may be added to create pores in the film to adjust the release pattern of sustained-release formulations. Coloring agents (e.g., titanium dioxide, kaolin) may be added to modify appearance.
Functional Category: Plasticizer
 Description:
A plasticizer is a low molecular weight substance that, when added to another material—usually a polymer—makes the latter flexible, resilient, and easier to handle. Modern plasticizers are synthetic organic chemicals, the majority of which are esters such as citrates and phthalates. They are key components that determine the physical properties of polymeric pharmaceutical systems such as tablet film coatings and capsule shells. Description:
A plasticizer is a low molecular weight substance that, when added to another material—usually a polymer—makes the latter flexible, resilient, and easier to handle. Modern plasticizers are synthetic organic chemicals, the majority of which are esters such as citrates and phthalates. They are key components that determine the physical properties of polymeric pharmaceutical systems such as tablet film coatings and capsule shells.
 Functional Mechanism:
Plasticizers function by increasing the intermolecular and intramolecular mobility of the macromolecules that comprise polymeric materials. They achieve this by interfering with the normal intermolecular and intramolecular bonding mechanisms in such systems. The most effective plasticizers exert their effect at low concentrations, typically less than 5% w/w. Plasticizers commonly are added to film coatings (aqueous and nonaqueous systems) and capsule shells (hard and soft varieties) to improve their workability and mechanical ruggedness. Without the addition of plasticizers, such materials can split or fracture prematurely. Plasticizers also are added to semisolid pharmaceutical preparations such as creams and ointments to enhance their rheological properties. Functional Mechanism:
Plasticizers function by increasing the intermolecular and intramolecular mobility of the macromolecules that comprise polymeric materials. They achieve this by interfering with the normal intermolecular and intramolecular bonding mechanisms in such systems. The most effective plasticizers exert their effect at low concentrations, typically less than 5% w/w. Plasticizers commonly are added to film coatings (aqueous and nonaqueous systems) and capsule shells (hard and soft varieties) to improve their workability and mechanical ruggedness. Without the addition of plasticizers, such materials can split or fracture prematurely. Plasticizers also are added to semisolid pharmaceutical preparations such as creams and ointments to enhance their rheological properties.
 Physical Properties:
The most common plasticizers are low molecular weight (< 500 Da) solids or liquids. They typically have low melting points (< 100 Physical Properties:
The most common plasticizers are low molecular weight (< 500 Da) solids or liquids. They typically have low melting points (< 100 ) and can be volatile (i.e., exert an appreciable vapor pressure) at ambient temperature. Plasticizers can significantly reduce the glass transition temperature of the system to which they are added. ) and can be volatile (i.e., exert an appreciable vapor pressure) at ambient temperature. Plasticizers can significantly reduce the glass transition temperature of the system to which they are added.
 Chemical Properties:
As noted, many modern plasticizers are synthetic esters such as citrates and phthalates. Traditional pharmaceutical plasticizers include oils, sugars, and their derivatives. Chemical Properties:
As noted, many modern plasticizers are synthetic esters such as citrates and phthalates. Traditional pharmaceutical plasticizers include oils, sugars, and their derivatives.
 Other Information:
The choice of an appropriate plasticizer often is guided by reference to its “solubility parameter”, which is related to its cohesive energy density. Solubility parameter values for many common materials are tabulated in standard reference texts. To ensure maximum effectiveness, the solubility parameter of the plasticizer and the polymeric system being plasticized should be matched as closely as possible. Other Information:
The choice of an appropriate plasticizer often is guided by reference to its “solubility parameter”, which is related to its cohesive energy density. Solubility parameter values for many common materials are tabulated in standard reference texts. To ensure maximum effectiveness, the solubility parameter of the plasticizer and the polymeric system being plasticized should be matched as closely as possible.
ORAL LIQUIDS
Functional Category: pH Modifier (Acidifying/Alkalizing/Buffering Agent)
 Description:
The hydrogen ion concentration, [H+], in an aqueous solution is expressed as pH = Description:
The hydrogen ion concentration, [H+], in an aqueous solution is expressed as pH =  log(H+). The pH of pure water is 7 at 25 log(H+). The pH of pure water is 7 at 25 . An aqueous solution is acidic at pH < 7 and alkaline at pH > 7. An acid may be added to acidify a solution. Similarly, a base may be used to alkalize a solution. A buffer is a weak acid (or base) and its salt. When a buffer is present in a solution, the addition of small quantities of strong acid or base leads to only a small change in solution pH. Buffer capacity is influenced by salt/acid (or base/salt) ratio and total concentration of acid (or base) and salt. The pH of pharmaceutical solutions typically is controlled using acidifying/alkalizing and buffering agents to (1) maintain a pH close to that of relevant body fluid to avoid irritation; (2) improve drug stability that is pH dependent; (3) control equilibrium solubility of weak acids or bases; and (4) maintain a consistent ionization state of molecules during chemical analysis, e.g., high-performance liquid chromatography (HPLC). . An aqueous solution is acidic at pH < 7 and alkaline at pH > 7. An acid may be added to acidify a solution. Similarly, a base may be used to alkalize a solution. A buffer is a weak acid (or base) and its salt. When a buffer is present in a solution, the addition of small quantities of strong acid or base leads to only a small change in solution pH. Buffer capacity is influenced by salt/acid (or base/salt) ratio and total concentration of acid (or base) and salt. The pH of pharmaceutical solutions typically is controlled using acidifying/alkalizing and buffering agents to (1) maintain a pH close to that of relevant body fluid to avoid irritation; (2) improve drug stability that is pH dependent; (3) control equilibrium solubility of weak acids or bases; and (4) maintain a consistent ionization state of molecules during chemical analysis, e.g., high-performance liquid chromatography (HPLC).
 Functional Mechanism:
The ionization equilibria of weak bases, weak acids, and water are the key to the functions of acidifying, alkalizing, and buffering agents. The autoprotolytic reaction of water can be expressed as
The autoprotolysis constant (or ion product) of water is Kw = 1 × 10 Functional Mechanism:
The ionization equilibria of weak bases, weak acids, and water are the key to the functions of acidifying, alkalizing, and buffering agents. The autoprotolytic reaction of water can be expressed as
The autoprotolysis constant (or ion product) of water is Kw = 1 × 10 14 at 25 14 at 25 and varies significantly with temperature. Because the concentrations of hydrogen and hydroxyl ions in pure water are equal, each has the value of approximately 1 × 10 and varies significantly with temperature. Because the concentrations of hydrogen and hydroxyl ions in pure water are equal, each has the value of approximately 1 × 10 7 mole/L, leading to the neutral pH of 7 at 25 7 mole/L, leading to the neutral pH of 7 at 25 . When an acid, base, or salt of weak acid (or base) is added, the ionization equilibrium of water is shifted so that [H+][OH . When an acid, base, or salt of weak acid (or base) is added, the ionization equilibrium of water is shifted so that [H+][OH ] remains constant, thus resulting in a solution pH that is different from 7. ] remains constant, thus resulting in a solution pH that is different from 7.
 Physical Properties:
The ionization equilibrium of a weak acid, HA, can be written as
The ionization constant of a weak acid (or conjugate acid of a base) is commonly expressed as pKa = Physical Properties:
The ionization equilibrium of a weak acid, HA, can be written as
The ionization constant of a weak acid (or conjugate acid of a base) is commonly expressed as pKa =  log(Ka), where Ka = [H3O+][A log(Ka), where Ka = [H3O+][A ]/[HA]. A lower pKa corresponds to a stronger acid. Similarly, the ionization constant of a weak base (or conjugate base of an acid) is expressed as pKb = ]/[HA]. A lower pKa corresponds to a stronger acid. Similarly, the ionization constant of a weak base (or conjugate base of an acid) is expressed as pKb =  log(Kb). The ionization equilibrium of water (pKa + pKb = pKw) equals 14 at 25 log(Kb). The ionization equilibrium of water (pKa + pKb = pKw) equals 14 at 25 . Buffers and pH modifiers influence solution osmolality, osmolarity, and water conductivity. . Buffers and pH modifiers influence solution osmolality, osmolarity, and water conductivity.
 Chemical Properties:
When used in chemical analysis, buffers must be chemically compatible with the reagents and test substance. Buffers, when used in physiological systems, should not interfere with pharmacological activity of the medicament or normal function of the organism. Chemical Properties:
When used in chemical analysis, buffers must be chemically compatible with the reagents and test substance. Buffers, when used in physiological systems, should not interfere with pharmacological activity of the medicament or normal function of the organism.
Functional Category: Wetting and/or Solubilizing Agent
 Description:
Solubilizers can be used to dissolve insoluble molecules. They function by facilitating spontaneous phase transfer to yield a thermodynamically stable solution. A number of solubilizers are available commercially. Acceptable solubilizers for pharmaceutical applications have been fully evaluated in animals for safety and toxicology. Description:
Solubilizers can be used to dissolve insoluble molecules. They function by facilitating spontaneous phase transfer to yield a thermodynamically stable solution. A number of solubilizers are available commercially. Acceptable solubilizers for pharmaceutical applications have been fully evaluated in animals for safety and toxicology.
 Functional Mechanism:
Solubilizers comprise a variety of different chemical structures/classes. Some solubilizers may have unique chemical structures. For example, a hydrophilic moiety may be tethered with a hydrophobic moiety to yield distinct micelle shapes and morphologies in water, thus facilitating solubilization. The mechanism of solubilization often is associated with a favorable interaction of the insoluble agent and the interior core of the solubilizer assembly (e.g. micelles). In other cases, unique hydrophobic sites that are capable of forming inclusion complexes are present. Other types of solubilizers utilize a range of polymeric chains that interact with hydrophobic molecules to increase solubility by dissolving the insoluble agent into the polymeric chains. Functional Mechanism:
Solubilizers comprise a variety of different chemical structures/classes. Some solubilizers may have unique chemical structures. For example, a hydrophilic moiety may be tethered with a hydrophobic moiety to yield distinct micelle shapes and morphologies in water, thus facilitating solubilization. The mechanism of solubilization often is associated with a favorable interaction of the insoluble agent and the interior core of the solubilizer assembly (e.g. micelles). In other cases, unique hydrophobic sites that are capable of forming inclusion complexes are present. Other types of solubilizers utilize a range of polymeric chains that interact with hydrophobic molecules to increase solubility by dissolving the insoluble agent into the polymeric chains.
 Physical Properties:
Solubilizers are solid, liquid, or waxy materials. Their physical properties depend on their chemical structures. The physical properties and performance of the solubilizers, however, depend on the surface-active properties of the solubilizers and on the hydrophilic–lipophilic balance (HLB). Solubilizers with lower HLB values behave as emulsifiers, and those with higher HLB values behave as solubilizers. For example, sodium lauryl sulfate (HLB 40) is hydrophilic and highly water soluble and, upon dispersion in water, spontaneously forms micelles. Physical Properties:
Solubilizers are solid, liquid, or waxy materials. Their physical properties depend on their chemical structures. The physical properties and performance of the solubilizers, however, depend on the surface-active properties of the solubilizers and on the hydrophilic–lipophilic balance (HLB). Solubilizers with lower HLB values behave as emulsifiers, and those with higher HLB values behave as solubilizers. For example, sodium lauryl sulfate (HLB 40) is hydrophilic and highly water soluble and, upon dispersion in water, spontaneously forms micelles.
The unique hydrophilicity and hydrophobicity properties of solubilizers are characterized by their aggregate numbers or critical micelle concentrations (CMC). The CMC value is unique to an individual solubilizer bearing hydrophilic, lipophilic, and/or hydrophobic chains. CMC is a measure of the concentration at which the surface-active molecule aggregates and solubilizes the solute by incorporating part into the hydrophobic interior and accommodating the rest in the hydrophilic exterior aqueous layer. Such interactions with the insoluble molecule further stabilize the molecules in the entire assemblies without precipitation to yield a continuous solution.
 Chemical Properties:
The chemical and surface-active properties depend on the structures of the solubilizers. Because of the complex nature of solute–solvent–solubilizer interactions, pharmaceutical scientists must carefully consider, identify, and control the critical material attributes of solubilizers. Chemical Properties:
The chemical and surface-active properties depend on the structures of the solubilizers. Because of the complex nature of solute–solvent–solubilizer interactions, pharmaceutical scientists must carefully consider, identify, and control the critical material attributes of solubilizers.
Functional Category: Antimicrobial Preservative
 Description:
Antimicrobial preservatives are used to kill or prevent growth of bacteria, yeast, and mold in the dosage form. Description:
Antimicrobial preservatives are used to kill or prevent growth of bacteria, yeast, and mold in the dosage form.
 Functional Mechanism:
Preservatives work by a variety of mechanisms to control microbes. Most of them work at the cell membrane, causing membrane damage and cell leakage. Other modes of action include transport inhibition, protein precipitation, and proton-conducting uncoupling. Some preservatives are -cidal (kill bacteria or yeast and mold); some are -static (inhibit growth of microorganisms); and others are sporicidal (kill spores). Several of the preservatives can act synergistically (e.g., combinations of parabens). Functional Mechanism:
Preservatives work by a variety of mechanisms to control microbes. Most of them work at the cell membrane, causing membrane damage and cell leakage. Other modes of action include transport inhibition, protein precipitation, and proton-conducting uncoupling. Some preservatives are -cidal (kill bacteria or yeast and mold); some are -static (inhibit growth of microorganisms); and others are sporicidal (kill spores). Several of the preservatives can act synergistically (e.g., combinations of parabens).
 Physical Properties:
Antimicrobials generally are soluble in water at concentration ranges at which they are effective. The vapor pressure of these agents is important, especially if the dosage form is intended to be lyophilized or spray dried. Several of these agents are flammable. Understanding of an excipient's partition coefficient is important because partitioning of a preservative into an oil phase will diminish the preservative's concentration in the aqueous phase, which in turn can reduce its value as a preservative. Physical Properties:
Antimicrobials generally are soluble in water at concentration ranges at which they are effective. The vapor pressure of these agents is important, especially if the dosage form is intended to be lyophilized or spray dried. Several of these agents are flammable. Understanding of an excipient's partition coefficient is important because partitioning of a preservative into an oil phase will diminish the preservative's concentration in the aqueous phase, which in turn can reduce its value as a preservative.
 Chemical Properties:
Phenolic preservatives can undergo oxidation and color formation. Incompatibilites of preservatives (cationic and anionic mixtures, adsorption to tubes or filters, binding to surfactants and proteins) should be taken into account during product development. Chemical Properties:
Phenolic preservatives can undergo oxidation and color formation. Incompatibilites of preservatives (cationic and anionic mixtures, adsorption to tubes or filters, binding to surfactants and proteins) should be taken into account during product development.
 Other Information:
Be aware of safety and labeling requirements, specifically for benzalkonium chloride (eye and skin irritation), benzoic acid and benzoate salts (risk of jaundice in newborn babies), benzyl alcohol (should not be given to premature babies or neonates and may cause allergic reactions in children aged 3 years or less), bronopol (may cause skin reaction), chlorocresol (allergic reactions), organic mercury compounds (allergic reaction), parabens (allergic reactions), and sorbic acid and salts (skin reactions). Because of the risk of organic mercury toxicity, thimerosal should not be used. Use of preservative is contraindicated in parenteral products in which the fill volume is greater than 30 mL or comes in contact with cerebrospinal fluid. Antioxidants and chelating agents tend to potentiate antimicrobial efficacy. Other Information:
Be aware of safety and labeling requirements, specifically for benzalkonium chloride (eye and skin irritation), benzoic acid and benzoate salts (risk of jaundice in newborn babies), benzyl alcohol (should not be given to premature babies or neonates and may cause allergic reactions in children aged 3 years or less), bronopol (may cause skin reaction), chlorocresol (allergic reactions), organic mercury compounds (allergic reaction), parabens (allergic reactions), and sorbic acid and salts (skin reactions). Because of the risk of organic mercury toxicity, thimerosal should not be used. Use of preservative is contraindicated in parenteral products in which the fill volume is greater than 30 mL or comes in contact with cerebrospinal fluid. Antioxidants and chelating agents tend to potentiate antimicrobial efficacy.
Functional Category: Chelating and/or Complexing Agents
 Description:
Chelating/complexing agents form soluble complex molecules with certain metal ions (e.g., copper, iron, manganese, lead, and calcium) and essentially remove the ions from solution to minimize or eliminate their ability to react with other elements and/or to precipitate. The agents are used in pharmaceuticals (oral, parenteral, and topical formulations), cosmetics, and foods to sequester ions from solution and to form stable complexes. Chelating agents are also referred to as chelants, chelators, or sequestering agents. Description:
Chelating/complexing agents form soluble complex molecules with certain metal ions (e.g., copper, iron, manganese, lead, and calcium) and essentially remove the ions from solution to minimize or eliminate their ability to react with other elements and/or to precipitate. The agents are used in pharmaceuticals (oral, parenteral, and topical formulations), cosmetics, and foods to sequester ions from solution and to form stable complexes. Chelating agents are also referred to as chelants, chelators, or sequestering agents.
 Functional Mechanism:
Chelating/complexing agents are used to sequester undesirable metal ions from solution. Their chemical structure acts as a “claw” to associate with the metal atom by forming a heterocyclic ring structure. Complexing agents function similarly but mechanistically do not (by definition) require a two-point claw structure because they can associate via one or more binding sites. All chelating agents are complexing agents, but not all complexing agents are chelating agents. As excipients, chelating agents are used as antioxidant synergists, antimicrobial synergists, and water softeners. By “removing” metal ions from solution, chelating agents reduce the propensity for oxidative reactions. Chelating agents also have the ability to enhance antimicrobial effectiveness by forming a metal-ion-deficient environment that otherwise could feed microbial growth. Functional Mechanism:
Chelating/complexing agents are used to sequester undesirable metal ions from solution. Their chemical structure acts as a “claw” to associate with the metal atom by forming a heterocyclic ring structure. Complexing agents function similarly but mechanistically do not (by definition) require a two-point claw structure because they can associate via one or more binding sites. All chelating agents are complexing agents, but not all complexing agents are chelating agents. As excipients, chelating agents are used as antioxidant synergists, antimicrobial synergists, and water softeners. By “removing” metal ions from solution, chelating agents reduce the propensity for oxidative reactions. Chelating agents also have the ability to enhance antimicrobial effectiveness by forming a metal-ion-deficient environment that otherwise could feed microbial growth.
 Physical Properties:
Chelating and complexing agents are freely soluble in water. Various salt (disodium and calcium disodium) and hydrated forms (anhydrous, dihydrate, and trihydrate) of edetic acid exist. Edetic acid and its derivatives appear as white to off-white crystalline solids. Oxyquinoline sulfate appears as a pale yellow, crystalline powder. USP–NF recognizes that chelating/complexing agents are stable below 100 Physical Properties:
Chelating and complexing agents are freely soluble in water. Various salt (disodium and calcium disodium) and hydrated forms (anhydrous, dihydrate, and trihydrate) of edetic acid exist. Edetic acid and its derivatives appear as white to off-white crystalline solids. Oxyquinoline sulfate appears as a pale yellow, crystalline powder. USP–NF recognizes that chelating/complexing agents are stable below 100 , but dehydration and/or decomposition can occur at higher temperatures. Chelating agents exhibit different degrees of hygroscopicity. Because low proportions of chelating agents are used in formulations (typically not more than 0.2%), they are not expected to significantly affect the bulk solid mechanical and flow properties of solid formulations. Because these agents are used in very low levels, their particle size distribution is important to enable acceptable dosage form content uniformity. , but dehydration and/or decomposition can occur at higher temperatures. Chelating agents exhibit different degrees of hygroscopicity. Because low proportions of chelating agents are used in formulations (typically not more than 0.2%), they are not expected to significantly affect the bulk solid mechanical and flow properties of solid formulations. Because these agents are used in very low levels, their particle size distribution is important to enable acceptable dosage form content uniformity.
 Chemical Properties:
Chelating/complexing agents complex with metal ions via any combination of ionic and covalent bonds. Dilute aqueous solutions may be neutral, acidic, or alkaline. Edetic acid and its salts are incompatible with strong oxidizers, strong bases, and polyvalent metal ions (e.g., copper and nickel). Specific agents are selected for a formulation based on their solubility, affinity for the target metal ion, and stability. Edetate salts are more soluble than the free acid. Unlike other edetate salts and the free acid, edetate calcium disodium does not sequester calcium and therefore is preferred to prevent hypocalcemia. It is also preferred to chelate heavy metals with the release of calcium ions. Alternatively, disodium edetate can be used to treat hypercalcemia. Edetic acid will decarboxylate if heated above 150 Chemical Properties:
Chelating/complexing agents complex with metal ions via any combination of ionic and covalent bonds. Dilute aqueous solutions may be neutral, acidic, or alkaline. Edetic acid and its salts are incompatible with strong oxidizers, strong bases, and polyvalent metal ions (e.g., copper and nickel). Specific agents are selected for a formulation based on their solubility, affinity for the target metal ion, and stability. Edetate salts are more soluble than the free acid. Unlike other edetate salts and the free acid, edetate calcium disodium does not sequester calcium and therefore is preferred to prevent hypocalcemia. It is also preferred to chelate heavy metals with the release of calcium ions. Alternatively, disodium edetate can be used to treat hypercalcemia. Edetic acid will decarboxylate if heated above 150 . .
Functional Category: Antioxidant
 Description:
This category applies to antioxidants used as in vitro stabilizers of pharmaceutical preparations to mitigate oxidative processes. Antioxidants used for their biological activity in vivo may be regarded as active ingredients with therapeutic effects and are not discussed. Antioxidants delay the onset and/or significantly reduce the rate of complex oxidative reactions that could otherwise have a detrimental impact on the drug substance. Antioxidants also can be considered for protecting nonactive components like unsaturated oils, pegylated lipids, flavors, and essential oils. Thus antioxidants preserve the overall integrity of the dosage form against oxidative stress. Antioxidants are most effective when incorporated in the formula to prevent or delay the onset of chain reactions and to inhibit free radicals and hydroperoxides from engaging in the cascading processes described above. Effective application of antioxidants and evaluation of their efficacy necessitate an understanding of oxidative mechanisms and the nature of the byproducts they generate. Autoxidation is initiated when oxygen reacts with a substrate to form highly reactive species known as free radicals (RH ® R·). After “initiation” the free radicals in the presence of oxygen can trigger chain reactions (R·+ O2 ® ROO·and ROO·+ RH ® R·+ ROOH) to form peroxy radicals, hydroperoxides, and new alkyl radicals that can initiate and then propagate their own chain reactions. The cascading reactions during the propagation phase can be accelerated by heat, light, and metal catalysts. In the presence of trace amounts of metal catalysts (Cu+, Cu2+, Fe2+, and Fe3+), hydroperoxides (ROOH) readily decompose to RO·and ROO·and can subsequently trigger reactions with the API and/or the excipients (e.g., hydrocarbons) to form hydroxyl acids, keto acids, and aldehydes that can have further undesirable effects. Note that hydroperoxides are not solely the reaction products of oxidative mechanisms within a formulation. Residual amounts of hydroperoxides can also be found in commonly used excipients like polyethylene glycols (PEG), polyvinlypyrrolidone (PVP), and polysorbates. The initiation phase generally is slow and has limited impact on the quality of the finished product. The propagation phase, in contrast, involves rapid, irreversible degradation of chemical species. Description:
This category applies to antioxidants used as in vitro stabilizers of pharmaceutical preparations to mitigate oxidative processes. Antioxidants used for their biological activity in vivo may be regarded as active ingredients with therapeutic effects and are not discussed. Antioxidants delay the onset and/or significantly reduce the rate of complex oxidative reactions that could otherwise have a detrimental impact on the drug substance. Antioxidants also can be considered for protecting nonactive components like unsaturated oils, pegylated lipids, flavors, and essential oils. Thus antioxidants preserve the overall integrity of the dosage form against oxidative stress. Antioxidants are most effective when incorporated in the formula to prevent or delay the onset of chain reactions and to inhibit free radicals and hydroperoxides from engaging in the cascading processes described above. Effective application of antioxidants and evaluation of their efficacy necessitate an understanding of oxidative mechanisms and the nature of the byproducts they generate. Autoxidation is initiated when oxygen reacts with a substrate to form highly reactive species known as free radicals (RH ® R·). After “initiation” the free radicals in the presence of oxygen can trigger chain reactions (R·+ O2 ® ROO·and ROO·+ RH ® R·+ ROOH) to form peroxy radicals, hydroperoxides, and new alkyl radicals that can initiate and then propagate their own chain reactions. The cascading reactions during the propagation phase can be accelerated by heat, light, and metal catalysts. In the presence of trace amounts of metal catalysts (Cu+, Cu2+, Fe2+, and Fe3+), hydroperoxides (ROOH) readily decompose to RO·and ROO·and can subsequently trigger reactions with the API and/or the excipients (e.g., hydrocarbons) to form hydroxyl acids, keto acids, and aldehydes that can have further undesirable effects. Note that hydroperoxides are not solely the reaction products of oxidative mechanisms within a formulation. Residual amounts of hydroperoxides can also be found in commonly used excipients like polyethylene glycols (PEG), polyvinlypyrrolidone (PVP), and polysorbates. The initiation phase generally is slow and has limited impact on the quality of the finished product. The propagation phase, in contrast, involves rapid, irreversible degradation of chemical species.
 Functional Mechanism:
Antioxidants can be grouped by their mode of action. Phenolic antioxidants that block free radical chain reactions are also known as true or primary antioxidants. This group consists of monohydroxy or polyhydroxy phenol compounds with ring substitutions. They have very low activation energy to donate hydrogen atom(s) in exchange for the radical electrons that are rapidly delocalized by free radicals. By accepting the radical electrons they stabilize free radicals. The reaction yields antioxidant free radicals that can also react with lipid free radicals to form other stable compounds. Thus they can block oxidative chain reactions both in the initiation and propagation stages. Because of their solubility behavior, phenolic antioxidants are most effective in protecting oils and oil-soluble actives against oxidative stress. Reducing agents generally are water-soluble antioxidants (e.g., l-ascorbic acid) with lower redox potential than the drug or the excipient they are protecting. They delay the onset and the rate of oxidative reactions by sacrificially reacting with oxygen and other reactive species. The oxygen-scavenging potential of the reducing agents may be sensitive to pH and can also be negatively affected in the presence of trace metals. Chelating agents bind with free metals (Cu+, Cu2+, Fe2+, and Fe3+) that may be present in trace amounts in the formulation. The newly formed complex ions are nonreactive. Chelating agents therefore remove the capacity of the metal catalysts to participate in oxidative reactions that occur during the propagation stage. Functional Mechanism:
Antioxidants can be grouped by their mode of action. Phenolic antioxidants that block free radical chain reactions are also known as true or primary antioxidants. This group consists of monohydroxy or polyhydroxy phenol compounds with ring substitutions. They have very low activation energy to donate hydrogen atom(s) in exchange for the radical electrons that are rapidly delocalized by free radicals. By accepting the radical electrons they stabilize free radicals. The reaction yields antioxidant free radicals that can also react with lipid free radicals to form other stable compounds. Thus they can block oxidative chain reactions both in the initiation and propagation stages. Because of their solubility behavior, phenolic antioxidants are most effective in protecting oils and oil-soluble actives against oxidative stress. Reducing agents generally are water-soluble antioxidants (e.g., l-ascorbic acid) with lower redox potential than the drug or the excipient they are protecting. They delay the onset and the rate of oxidative reactions by sacrificially reacting with oxygen and other reactive species. The oxygen-scavenging potential of the reducing agents may be sensitive to pH and can also be negatively affected in the presence of trace metals. Chelating agents bind with free metals (Cu+, Cu2+, Fe2+, and Fe3+) that may be present in trace amounts in the formulation. The newly formed complex ions are nonreactive. Chelating agents therefore remove the capacity of the metal catalysts to participate in oxidative reactions that occur during the propagation stage.
The utility of antioxidants can be maximized by synergistic use of one or two primary antioxidants along with reducing and chelating agents. The combined effect is often greater than the sum of the individual effects of each antioxidant (synergistic effect).
 Physical Properties:
Solubility of the antioxidant should be greatest in the formulation phase (oily, aqueous, or emulsion interface) where the drug substance is most soluble. The temperature at which the antioxidant decomposes is critical for autoclaved preparations where loss of antioxidant activity may occur. Stability of the antioxidant also must be considered and may be a function of pH and processing conditions. Metal ions may react with propyl gallate to form colored complexes. At alkaline pH, certain proteins and sodium salts may bring about discoloration of tert-butylhydroquinone (TBHQ). Physical Properties:
Solubility of the antioxidant should be greatest in the formulation phase (oily, aqueous, or emulsion interface) where the drug substance is most soluble. The temperature at which the antioxidant decomposes is critical for autoclaved preparations where loss of antioxidant activity may occur. Stability of the antioxidant also must be considered and may be a function of pH and processing conditions. Metal ions may react with propyl gallate to form colored complexes. At alkaline pH, certain proteins and sodium salts may bring about discoloration of tert-butylhydroquinone (TBHQ).
 Chemical Properties:
Activation energy, oxidation-reduction potential, stability at different formulation (e.g., pH), and processing (e.g., heat) conditions are important chemical properties. If the dosage form's expected shelf life depends on the antioxidant's function, the concentration must be factored in and periodically assessed to ensure that a sufficient amount of antioxidant remains but does not exceed safety limits. Chemical Properties:
Activation energy, oxidation-reduction potential, stability at different formulation (e.g., pH), and processing (e.g., heat) conditions are important chemical properties. If the dosage form's expected shelf life depends on the antioxidant's function, the concentration must be factored in and periodically assessed to ensure that a sufficient amount of antioxidant remains but does not exceed safety limits.
Functional Category: Sweetening Agent
 Description:
Sweetening agents are used to sweeten oral dosage forms and to mask unpleasant flavors. Description:
Sweetening agents are used to sweeten oral dosage forms and to mask unpleasant flavors.
 Functional Mechanism:
Sweetening agents bind to receptors on the tongue that are responsible for the sensation of sweetness. The longer the sweetener molecule remains attached to the receptor, the sweeter the substance is perceived to be. The standard for sweetness is sucrose. Functional Mechanism:
Sweetening agents bind to receptors on the tongue that are responsible for the sensation of sweetness. The longer the sweetener molecule remains attached to the receptor, the sweeter the substance is perceived to be. The standard for sweetness is sucrose.
 Physical Properties:
The primary physical properties relevant to sweeteners relate to their compatibility with the other ingredients in the formulation (e.g., acidic ingredients), processing conditions (e.g., heating), particle size and distribution, moisture content, isomerism, sweetness, and taste-masking capability. These properties may be formulation dependent. Physical Properties:
The primary physical properties relevant to sweeteners relate to their compatibility with the other ingredients in the formulation (e.g., acidic ingredients), processing conditions (e.g., heating), particle size and distribution, moisture content, isomerism, sweetness, and taste-masking capability. These properties may be formulation dependent.
 Chemical Properties:
Sweeteners can be divided into three main groups: sugars (which have a ring structure), sugar alcohols (sugars that do not have a ring structure), and artificial sweeteners. All sweeteners are water soluble. The stability of many sweeteners is affected by pH and other ingredients in the formulation. Some sweeteners may catalyze the degradation of some active ingredients, especially in liquids and in cases in which the manufacturing processes involve heating. Chemical Properties:
Sweeteners can be divided into three main groups: sugars (which have a ring structure), sugar alcohols (sugars that do not have a ring structure), and artificial sweeteners. All sweeteners are water soluble. The stability of many sweeteners is affected by pH and other ingredients in the formulation. Some sweeteners may catalyze the degradation of some active ingredients, especially in liquids and in cases in which the manufacturing processes involve heating.
 Other Information:
Products that contain aspartame must include a warning on the label stating that the product contains phenylalanine. Sugar alcohols have a glycemic index well below that of glucose. However, sorbitol is slowly metabolized to fructose and glucose, which raises blood sugar levels. Sugar alcohols in quantities generally greater than 20 g/day act as an osmotic laxative, especially when they are contained in a liquid formulation. Preservative systems should be carefully chosen to avoid incompatibility with the sweetener; some sweeteners are incompatible with certain preservatives. Other Information:
Products that contain aspartame must include a warning on the label stating that the product contains phenylalanine. Sugar alcohols have a glycemic index well below that of glucose. However, sorbitol is slowly metabolized to fructose and glucose, which raises blood sugar levels. Sugar alcohols in quantities generally greater than 20 g/day act as an osmotic laxative, especially when they are contained in a liquid formulation. Preservative systems should be carefully chosen to avoid incompatibility with the sweetener; some sweeteners are incompatible with certain preservatives.
SEMISOLIDS, TOPICALS, AND SUPPOSITORIES
Functional Category: Suppository Base
 Description:
Suppository bases are used in the manufacture of suppositories (for rectal administration) and pessaries (for vaginal administration). They can be hydrophobic or hydrophilic. Description:
Suppository bases are used in the manufacture of suppositories (for rectal administration) and pessaries (for vaginal administration). They can be hydrophobic or hydrophilic.
 Functional Mechanism:
Suppositories should melt at just below body temperature (37 Functional Mechanism:
Suppositories should melt at just below body temperature (37 ), thereby allowing the drug to be released either by erosion and partition if the drug is dissolved in the base or by erosion and dissolution if the drug is suspended in the base. Hard fat suppository bases melt at approximately body temperature. Hydrophilic suppository bases also melt at body temperature and typically also dissolve or disperse in aqueous media. Thus release takes place via a combination of erosion and dissolution. ), thereby allowing the drug to be released either by erosion and partition if the drug is dissolved in the base or by erosion and dissolution if the drug is suspended in the base. Hard fat suppository bases melt at approximately body temperature. Hydrophilic suppository bases also melt at body temperature and typically also dissolve or disperse in aqueous media. Thus release takes place via a combination of erosion and dissolution.
 Physical Properties:
The important physical characteristic of suppository bases is melting range. In general suppository bases melt between 27 Physical Properties:
The important physical characteristic of suppository bases is melting range. In general suppository bases melt between 27 and 45 and 45 . However, individual bases usually have a much narrower melting range within these temperature boundaries, typically 2 . However, individual bases usually have a much narrower melting range within these temperature boundaries, typically 2 –3 –3 . The choice of a particular melting range is dictated by the influence of the other formulation components on the melting range of the final product. . The choice of a particular melting range is dictated by the influence of the other formulation components on the melting range of the final product.
 Chemical Properties:
Hard fat suppository bases are mixtures of semisynthetic triglyceride esters of longer-chain fatty acids. They may contain varying proportions of mono- and di-glycerides and may also contain ethoxylated fatty acids. They are available in many different grades that are differentiated by melting range, hydroxyl number, acid value, iodine value, solidification range, and saponification number. Chemical Properties:
Hard fat suppository bases are mixtures of semisynthetic triglyceride esters of longer-chain fatty acids. They may contain varying proportions of mono- and di-glycerides and may also contain ethoxylated fatty acids. They are available in many different grades that are differentiated by melting range, hydroxyl number, acid value, iodine value, solidification range, and saponification number.
Hydrophilic suppository bases are mixtures of hydrophilic semisolid materials that in combination are solid at room temperature and yet release the drug by melting, erosion, and dissolution when administered to the patient. Hydrophilic suppository bases have much higher levels of hydroxyl groups or other hydrophilic groups than do hard fat suppository bases. Polyethylene glycols that show appropriate melting behavior are examples of hydrophilic suppository bases.
 Other Information:
Some materials included in suppositories based on hard fats have much higher melting ranges. These materials typically are microcrystalline waxes that help stabilize molten suspension formulations. Suppositories may also be manufactured from glycerinated gelatin. Other Information:
Some materials included in suppositories based on hard fats have much higher melting ranges. These materials typically are microcrystalline waxes that help stabilize molten suspension formulations. Suppositories may also be manufactured from glycerinated gelatin.
Functional Category: Suspending and/or Viscosity-Increasing Agent
 Description:
Suspending and/or viscosity-increasing agents are used in pharmaceutical formulations to stabilize disperse systems (e.g., suspensions or emulsions), to reduce the rate of solute or particulate transport, or to decrease the fluidity of liquid formulations. Description:
Suspending and/or viscosity-increasing agents are used in pharmaceutical formulations to stabilize disperse systems (e.g., suspensions or emulsions), to reduce the rate of solute or particulate transport, or to decrease the fluidity of liquid formulations.
 Functional Mechanism(s):
A number of mechanisms contribute to the dispersion stabilization or viscosity-increasing effect of these agents. The most common is the increase in viscosity–due to the entrapment of solvent by macromolecular chains or clay platelets—and the disruption of laminar flow. Other mechanisms include gel formation via a three-dimensional network of excipient molecules or particles throughout the solvent continuum and steric stabilization wherein the macromolecular or mineral component in the dispersion medium adsorbs to the surfaces of particles or droplets of the dispersed phase. The latter two mechanisms increase formulation stability by immobilizing the dispersed phase. Functional Mechanism(s):
A number of mechanisms contribute to the dispersion stabilization or viscosity-increasing effect of these agents. The most common is the increase in viscosity–due to the entrapment of solvent by macromolecular chains or clay platelets—and the disruption of laminar flow. Other mechanisms include gel formation via a three-dimensional network of excipient molecules or particles throughout the solvent continuum and steric stabilization wherein the macromolecular or mineral component in the dispersion medium adsorbs to the surfaces of particles or droplets of the dispersed phase. The latter two mechanisms increase formulation stability by immobilizing the dispersed phase.
 Physical Properties:
Each of the mechanisms—increased viscosity, gel formation, or steric stabilization—is a manifestation of the rheological character of the excipient. Because of the molecular weights and sizes of these excipients, the rheological profiles of their dispersions are non-Newtonian. Dispersions of these excipients display viscoelastic properties. The molecular weight distribution and polydispersity of the macromolecular excipients in this category are important criteria for their characterization. Physical Properties:
Each of the mechanisms—increased viscosity, gel formation, or steric stabilization—is a manifestation of the rheological character of the excipient. Because of the molecular weights and sizes of these excipients, the rheological profiles of their dispersions are non-Newtonian. Dispersions of these excipients display viscoelastic properties. The molecular weight distribution and polydispersity of the macromolecular excipients in this category are important criteria for their characterization.
 Chemical Properties:
The majority of the suspending and/or viscosity-increasing agents are (a) hydrophilic carbohydrate macromolecules (acacia, agar, alginic acid, carboxymethylcellulose, carrageenans, dextrin, gellan gum, guar gum, hydroxyethyl cellulose, hydroxypropyl cellulose, hypromellose, maltodextrin, methylcellulose, pectin, propylene glycol alginate, sodium alginate, starch, tragacanth, and xanthan gum) and (b) noncarbohydrate hydrophilic macromolecules, including gelatin, povidone carbomers, polyethylene oxide, and polyvinyl alcohol. Minerals (e.g., attapulgite, bentonite, magnesium aluminum silicate, and silicon dioxide) comprise the second-largest group of suspending and/or viscosity-increasing agents. Aluminum monostearate is the one non-macromolecular, non-mineral excipient in this functional category. It consists chiefly of variable proportions of aluminum monostearate and aluminum monopalmitate. Chemical Properties:
The majority of the suspending and/or viscosity-increasing agents are (a) hydrophilic carbohydrate macromolecules (acacia, agar, alginic acid, carboxymethylcellulose, carrageenans, dextrin, gellan gum, guar gum, hydroxyethyl cellulose, hydroxypropyl cellulose, hypromellose, maltodextrin, methylcellulose, pectin, propylene glycol alginate, sodium alginate, starch, tragacanth, and xanthan gum) and (b) noncarbohydrate hydrophilic macromolecules, including gelatin, povidone carbomers, polyethylene oxide, and polyvinyl alcohol. Minerals (e.g., attapulgite, bentonite, magnesium aluminum silicate, and silicon dioxide) comprise the second-largest group of suspending and/or viscosity-increasing agents. Aluminum monostearate is the one non-macromolecular, non-mineral excipient in this functional category. It consists chiefly of variable proportions of aluminum monostearate and aluminum monopalmitate.
 General Chapter:
The following general chapter may be useful in ensuring consistency in selected excipient functions: Viscosity General Chapter:
The following general chapter may be useful in ensuring consistency in selected excipient functions: Viscosity  911 911 . .
Functional Category: Ointment Base
 Description:
An ointment is a viscous semisolid preparation used topically on a variety of body surfaces. An ointment base is the major component of an ointment and controls its physical properties. Description:
An ointment is a viscous semisolid preparation used topically on a variety of body surfaces. An ointment base is the major component of an ointment and controls its physical properties.
 Functional Mechanism:
Ointment bases serve as vehicles for topical application of medicinal substances and also as emollients and protective agents for skin. Functional Mechanism:
Ointment bases serve as vehicles for topical application of medicinal substances and also as emollients and protective agents for skin.
 Physical Properties:
Ointment bases are liquids with a relatively high viscosity so that solids can be suspended as a stable mixture. Physical Properties:
Ointment bases are liquids with a relatively high viscosity so that solids can be suspended as a stable mixture.
Ointment bases are classified as (a) oleaginous ointment bases that are anhydrous, do not absorb water readily, are insoluble in water, and are not removable by water (e.g., petrolatum); (b) absorption ointment bases that are anhydrous and absorb some water but are insoluble in water and are not water removable (e.g., lanolin); (c) emulsion ointment bases that are water-in-oil or oil-in-water emulsions and are hydrous, absorb water, and are insoluble in water (e.g., creams of water, oils, waxes, and/or paraffins); and (d) water-soluble ointment bases that are anhydrous and absorb water and are soluble in water and are water removable (e.g., polyethylene glycol).
 Chemical Properties:
Ointment bases are selected to be inert and chemically stable. Chemical Properties:
Ointment bases are selected to be inert and chemically stable.
Functional Category: Stiffening Agent
 Description:
A stiffening agent is an agent or a mixture of agents that increases the viscosity or hardness of a preparation, especially in ointments and creams. Description:
A stiffening agent is an agent or a mixture of agents that increases the viscosity or hardness of a preparation, especially in ointments and creams.
 Functional Mechanism:
In general, stiffening agents are high melting point solids that increase the melting point of ointments or increase the consistency or body of creams. Stiffening agents can be either hydrophobic (e.g., hard fat or paraffin) or hydrophilic (e.g., polyethylene glycol, high molecular weight). Functional Mechanism:
In general, stiffening agents are high melting point solids that increase the melting point of ointments or increase the consistency or body of creams. Stiffening agents can be either hydrophobic (e.g., hard fat or paraffin) or hydrophilic (e.g., polyethylene glycol, high molecular weight).
 Physical Properties:
The primary physical property relevant to stiffening agents is their high melting point or melting range. Typical melting ranges for stiffening agents range from 43 Physical Properties:
The primary physical property relevant to stiffening agents is their high melting point or melting range. Typical melting ranges for stiffening agents range from 43 to 47 to 47 (cetyl esters wax), 53 (cetyl esters wax), 53 to 57 to 57 (glyceryl distearate), 69 (glyceryl distearate), 69 to 74 to 74 (glyceryl behenate), and 85 (glyceryl behenate), and 85 to 88 to 88 (castor oil, hydrogenated). (castor oil, hydrogenated).
 Chemical Properties:
Stiffening agents comprise a diverse group of materials that include glycerides of saturated fatty acids, solid aliphatic alcohols, esters of saturated fatty alcohols and saturated fatty acids, saturated hydrocarbons, blends of fatty alcohols and a polyoxyethylene derivative of a fatty acid ester of sorbitan, and higher ethylene glycol polymers. Chemical Properties:
Stiffening agents comprise a diverse group of materials that include glycerides of saturated fatty acids, solid aliphatic alcohols, esters of saturated fatty alcohols and saturated fatty acids, saturated hydrocarbons, blends of fatty alcohols and a polyoxyethylene derivative of a fatty acid ester of sorbitan, and higher ethylene glycol polymers.
 Other Information:
Some of the materials included as stiffening agents increase the water-holding capacity of ointments (e.g., petrolatum) or function as co-emulsifiers in creams. Examples include stearyl alcohol and cetyl alcohol. Other Information:
Some of the materials included as stiffening agents increase the water-holding capacity of ointments (e.g., petrolatum) or function as co-emulsifiers in creams. Examples include stearyl alcohol and cetyl alcohol.
Functional Category: Emollient
 Description:
Emollients are excipients used in topical preparations to impart lubrication, spreading ease, texture, and softening of the skin and to counter the potentially drying/irritating impact of surfactants on the skin. Description:
Emollients are excipients used in topical preparations to impart lubrication, spreading ease, texture, and softening of the skin and to counter the potentially drying/irritating impact of surfactants on the skin.
 Functional Mechanism:
Emollients help form a protective film and maintain the barrier function of the epidermis. Their efficacy may be described by three mechanisms of action: protection against the delipidizing and drying effects of surfactants, humectancy due to occlusion (by providing a layer of oil on the surface of the skin, emollients slow water loss and thus increase the moisture-retention capacity of the stratum corneum), and lubricity, adding slip or glide to the preparation. Functional Mechanism:
Emollients help form a protective film and maintain the barrier function of the epidermis. Their efficacy may be described by three mechanisms of action: protection against the delipidizing and drying effects of surfactants, humectancy due to occlusion (by providing a layer of oil on the surface of the skin, emollients slow water loss and thus increase the moisture-retention capacity of the stratum corneum), and lubricity, adding slip or glide to the preparation.
 Physical Properties:
Emollients impart one or more of the following attributes to a pharmaceutical preparation: spreading capacity, pleasant feel to the touch, softness of the skin, and indirect moisturization of the skin by preventing trans-epidermal water loss. Physical Properties:
Emollients impart one or more of the following attributes to a pharmaceutical preparation: spreading capacity, pleasant feel to the touch, softness of the skin, and indirect moisturization of the skin by preventing trans-epidermal water loss.
 Chemical Properties:
Emollients are either oils or are derived from components of oils as esters of fatty acids. Depending on the nature of its fatty acid ester, an emollient may be liquid, semisolid, or solid at room temperature. Generally, the higher the molecular weight of the fatty acid moiety (carbon chain length) the richer the feel and softness of the touch. Fluidity generally is imparted by shorter chain length and higher degree of unsaturation in the fatty acid moiety. The degree of branching of ester bonds also influences the emollient properties. Chemical Properties:
Emollients are either oils or are derived from components of oils as esters of fatty acids. Depending on the nature of its fatty acid ester, an emollient may be liquid, semisolid, or solid at room temperature. Generally, the higher the molecular weight of the fatty acid moiety (carbon chain length) the richer the feel and softness of the touch. Fluidity generally is imparted by shorter chain length and higher degree of unsaturation in the fatty acid moiety. The degree of branching of ester bonds also influences the emollient properties.
 General Chapter:
The following general chapter may be useful in ensuring consistency in selected excipient functions: Fats and Fixed Oils General Chapter:
The following general chapter may be useful in ensuring consistency in selected excipient functions: Fats and Fixed Oils  401 401 . .
PARENTERALS
Functional Category: Pharmaceutical Water
 Description:
Water is used as a solvent, vehicle, diluent, or filler for many drug products, especially those supplied in liquid form. These can include injectible drugs, ophthalmic drugs, oral solutions, inhalation solutions, and others. Water is also a vehicle for buffers and antimicrobial agents and is a volume expander for infusion solutions. Its use in dosage form preparation also can include granulation preparation for solid oral dosage forms and applications in the preparation of ointments and gels. Description:
Water is used as a solvent, vehicle, diluent, or filler for many drug products, especially those supplied in liquid form. These can include injectible drugs, ophthalmic drugs, oral solutions, inhalation solutions, and others. Water is also a vehicle for buffers and antimicrobial agents and is a volume expander for infusion solutions. Its use in dosage form preparation also can include granulation preparation for solid oral dosage forms and applications in the preparation of ointments and gels.
USP includes monographs for eight grades of pharmaceutical waters. One of these types of USP water is always the water of choice when pharmaceutical scientists prepare a pharmaceutical dosage form for human or animal use. However, USP also contains references to other types of water, such as distilled water, deionized water, and others according to specific use as summarized in general information chapter Water for Pharmaceutical Purposes  1231 1231 .
 Functional Mechanism:
A solvent is able to dissolve materials because it is able to disrupt the intermolecular attractive forces and to allow the individual molecules to become dispersed throughout the bulk solvent. Water is a favored solvent and vehicle in the majority of applications because it is easy to handle, safe, and inexpensive. Functional Mechanism:
A solvent is able to dissolve materials because it is able to disrupt the intermolecular attractive forces and to allow the individual molecules to become dispersed throughout the bulk solvent. Water is a favored solvent and vehicle in the majority of applications because it is easy to handle, safe, and inexpensive.
 Physical Properties:
Water is liquid at normal temperature and pressure. It forms ice at the freezing temperatures of 0 Physical Properties:
Water is liquid at normal temperature and pressure. It forms ice at the freezing temperatures of 0 or lower; and it vaporizes at a normal boiling temperature of 100 or lower; and it vaporizes at a normal boiling temperature of 100 , depending upon atmospheric pressure. Vaporized water in the form of steam is used for sterilization purposes because the latent heat of steam is significantly higher than that of boiling water. , depending upon atmospheric pressure. Vaporized water in the form of steam is used for sterilization purposes because the latent heat of steam is significantly higher than that of boiling water.
 Chemical Properties:
Water in its pure form is neutral in pH and has very low conductivity and total organic carbon (TOC). However, pH, conductivity, and TOC are affected by storage conditions and exposure of water to gases in the air. Exposure of water to atmospheric carbon dioxide lowers the pH of water. Storage of water in plastic containers may increase the TOC content of water over time. Water stored in glass containers may result in an increase in pH and conductivity of the water over time. Chemical Properties:
Water in its pure form is neutral in pH and has very low conductivity and total organic carbon (TOC). However, pH, conductivity, and TOC are affected by storage conditions and exposure of water to gases in the air. Exposure of water to atmospheric carbon dioxide lowers the pH of water. Storage of water in plastic containers may increase the TOC content of water over time. Water stored in glass containers may result in an increase in pH and conductivity of the water over time.
Functional Category: Diluent
 Description:
Diluents or bulking agents used in lyophilized pharmaceuticals include various saccharides, sugar alcohols, amino acids, and polymers. The primary functions of bulking agents are to provide a pharmaceutically elegant lyophized cake with non-collapsed structural integrity and to prevent drug loss due to blow-out. In addition, bulking agents are selected to facilitate efficient drying and to provide a physically and chemically stable formulation matrix. Frequently, complementary combinations of bulking agents are used to improve performance. Description:
Diluents or bulking agents used in lyophilized pharmaceuticals include various saccharides, sugar alcohols, amino acids, and polymers. The primary functions of bulking agents are to provide a pharmaceutically elegant lyophized cake with non-collapsed structural integrity and to prevent drug loss due to blow-out. In addition, bulking agents are selected to facilitate efficient drying and to provide a physically and chemically stable formulation matrix. Frequently, complementary combinations of bulking agents are used to improve performance.
So-called “good cake forming” excipients, such as mannitol, are frequently used because they tend to crystallize during freezing, thereby allowing efficient drying and the formation of a structurally robust cake. For some active ingredients, crystallization during lyophilization helps improve stability. Therefore the use of bulking agents that promote crystallization during lyophilization is important. Amino acids and cosolvents have been used to achieve this effect. Most biopolymer active ingredients remain amorphous upon freeze-drying, and bulking agents such as disaccharides may function as lyoprotectants by helping to maintain a stable amorphous phase during freezing and drying to prevent denaturation. Solubility enhancement of an insoluble crystalline active ingredient is sometimes achieved with the use of a biopolymer that enhances solubility or prevents crystallization of the active ingredient during lyophilization or subsequent reconstitution. Bulking agents are also selected on the bases of biocompatiability, buffering capability, and tonicity-modifying properties.
 Functional Mechanisms:
A bulking agent that readily crystallizes during lyophilization helps maintain the structural integrity of the cake formed during primary drying, thereby preventing macroscopic collapse and pharmaceutical inelegance. Microscopic collapse of amorphous components in the formulation may still occur (with some potentially undesirable results) but will not result in macroscopic collapse if the bulking agent properties and concentration are adequate. The bulking agent also should possess a high eutectic melting temperature with ice to permit relatively high primary drying temperatures with commensurate rapid and efficient drying. Functional Mechanisms:
A bulking agent that readily crystallizes during lyophilization helps maintain the structural integrity of the cake formed during primary drying, thereby preventing macroscopic collapse and pharmaceutical inelegance. Microscopic collapse of amorphous components in the formulation may still occur (with some potentially undesirable results) but will not result in macroscopic collapse if the bulking agent properties and concentration are adequate. The bulking agent also should possess a high eutectic melting temperature with ice to permit relatively high primary drying temperatures with commensurate rapid and efficient drying.
Lyoprotectant properties of lyophilization diluents (i.e., those that protect the drug substance during lyophilization) typically are achieved by the formation of a highly viscous glassy phase that includes the biopolymer drug substance in combination with low molecular weight amorphous saccharides such as sucrose, trehalose, or certain amino acids. A typical approach for protein pharmaceutical formulation is to combine a sugar alcohol that readily crystallizes and an amorphous diluent; this mixture acts as a lyoprotectant.
 Physical Properties:
Bulk agents are dissolved in aqueous solution before lyophilization. Therefore chemical purity and the absence of bioburden and pyrogenic materials are essential properties of the bulk excipient. However, the physical form and particle properties of the bulk excipient are generally not relevant to the final properties of the lyophilized formulation. Physical Properties:
Bulk agents are dissolved in aqueous solution before lyophilization. Therefore chemical purity and the absence of bioburden and pyrogenic materials are essential properties of the bulk excipient. However, the physical form and particle properties of the bulk excipient are generally not relevant to the final properties of the lyophilized formulation.
The physical properties that are essential to product performance during and after lyophilization include the glass transition temperature of the amorphous frozen concentrate before drying, the glass transition temperature of the final dried formulation cake, and the eutectic melting temperature of the crystalline bulking agent with ice. The glass transition temperature of the formulation depends on the glass transition temperatures of the individual components, concentrations, and interactions. Although approximations can be made based on reported transition temperatures for individual components, current practice includes the measurement of formulation glass transition temperatures by thermal analysis or freeze-drying microscopy.
The physical states of the bulking agent during and after lyophilization are important physical properties. Both formulation composition and processing parameters play roles in determining whether the bulking agent is amorphous or takes a specific crystalline form. For example, although mannitol is easily crystallized during lyophilization, it can also be amorphous based on formulation composition or can crystallize as a hydrate or metastable polymorph. Rate of freezing, drying temperatures, and annealing are among the important process parameters used to control the physical state of the formulation and its components. Moisture retention and adsorption after lyophilization also may contribute to formulation stability and performance.
 Chemical Properties:
Reactivity of the bulking agent with respect to other formulation components, especially the active ingredient, may be critical. Reducing sugars are well known to react with aromatic and aliphatic amines. Glycols may contain trace peroxide levels that can initiate oxidative degradation. The ability of saccharides and polyhydric alcohols to form hydrogen bonds to biopolymers may play a role in their lyoprotection effects. Chemical Properties:
Reactivity of the bulking agent with respect to other formulation components, especially the active ingredient, may be critical. Reducing sugars are well known to react with aromatic and aliphatic amines. Glycols may contain trace peroxide levels that can initiate oxidative degradation. The ability of saccharides and polyhydric alcohols to form hydrogen bonds to biopolymers may play a role in their lyoprotection effects.
Functional Category: Tonicity Agent
 Description:
To avoid crenation or hemolysis of red blood cells and to mitigate pain and discomfort if solutions are injected or introduced into the eyes and nose, solutions should be made isotonic. This requires that the effective osmotic pressure of solutions for injection is approximately the same as that in the blood. When drug products are prepared for administration to membranes such as eyes or nasal or vaginal tissues, solutions should be made isotonic with respect to these tissues. Description:
To avoid crenation or hemolysis of red blood cells and to mitigate pain and discomfort if solutions are injected or introduced into the eyes and nose, solutions should be made isotonic. This requires that the effective osmotic pressure of solutions for injection is approximately the same as that in the blood. When drug products are prepared for administration to membranes such as eyes or nasal or vaginal tissues, solutions should be made isotonic with respect to these tissues.
 Functional Mechanism:
Tonicity is equal to the sum of the concentrations of the solutes that have the capacity to exert an osmotic force across a membrane and thus reflects overall osmolality. Tonicity applies to the impermeant solutes within a solvent—in contrast to osmolarity, which takes into account both permeant and impermeant solutes. For example, urea is a permeant solute, meaning that it can pass through the cell membrane freely and is not factored when determining the tonicity of a solution. In contrast, sodium chloride is impermeant and cannot pass through a membrane without the help of a concentration gradient and will therefore contribute to a solution's tonicity. Functional Mechanism:
Tonicity is equal to the sum of the concentrations of the solutes that have the capacity to exert an osmotic force across a membrane and thus reflects overall osmolality. Tonicity applies to the impermeant solutes within a solvent—in contrast to osmolarity, which takes into account both permeant and impermeant solutes. For example, urea is a permeant solute, meaning that it can pass through the cell membrane freely and is not factored when determining the tonicity of a solution. In contrast, sodium chloride is impermeant and cannot pass through a membrane without the help of a concentration gradient and will therefore contribute to a solution's tonicity.
 Physical Properties:
Solutions of sodium chloride, dextrose, and Lactated Ringer's are common examples of pharmaceutical preparations that contain tonicity agents. Not all solutes contribute to the tonicity, which in general depends only on the number of solute particles present in a solution, not the kinds of solute particles. For example, mole for mole, sodium chloride solutions display a higher osmotic pressure than do glucose solutions of the same molar concentration. This is because when glucose dissolves it remains one particle, but when sodium chloride dissolves, it becomes two particles: Na+ and Cl Physical Properties:
Solutions of sodium chloride, dextrose, and Lactated Ringer's are common examples of pharmaceutical preparations that contain tonicity agents. Not all solutes contribute to the tonicity, which in general depends only on the number of solute particles present in a solution, not the kinds of solute particles. For example, mole for mole, sodium chloride solutions display a higher osmotic pressure than do glucose solutions of the same molar concentration. This is because when glucose dissolves it remains one particle, but when sodium chloride dissolves, it becomes two particles: Na+ and Cl . .
 Chemical Properties:
Tonicity agents may be present as ionic and/or nonionic types. Examples of ionic tonicity agents are alkali metal or earth metal halides such as CaCl2, KBr, KCl, LiCl, NaI, NaBr or NaCl, Na2SO4, or boric acid. Nonionic tonicity agents include glycerol, sorbitol, mannitol, propylene glycol, or dextrose. Chemical Properties:
Tonicity agents may be present as ionic and/or nonionic types. Examples of ionic tonicity agents are alkali metal or earth metal halides such as CaCl2, KBr, KCl, LiCl, NaI, NaBr or NaCl, Na2SO4, or boric acid. Nonionic tonicity agents include glycerol, sorbitol, mannitol, propylene glycol, or dextrose.
AEROSOLS
Functional Category: Propellant
 Description:
Propellants are compounds that are gaseous under ambient conditions. They are used in pharmaceuticals (nasal sprays and respiratory and topical formulations), cosmetics, and foods to provide force to expel contents from a container. Description:
Propellants are compounds that are gaseous under ambient conditions. They are used in pharmaceuticals (nasal sprays and respiratory and topical formulations), cosmetics, and foods to provide force to expel contents from a container.
 Functional Mechanism:
Propellant substances are low boiling point liquids that are relatively inert toward active ingredients and excipients. They can be characterized by three properties: whether they form a liquid phase at ambient temperatures and useful pressures, their solubility and/or miscibility in the rest of the formulation, and their flammability. Their performance is judged by their ability to provide adequate and predictable pressure throughout the usage life of the product. Functional Mechanism:
Propellant substances are low boiling point liquids that are relatively inert toward active ingredients and excipients. They can be characterized by three properties: whether they form a liquid phase at ambient temperatures and useful pressures, their solubility and/or miscibility in the rest of the formulation, and their flammability. Their performance is judged by their ability to provide adequate and predictable pressure throughout the usage life of the product.
Propellants that have both a liquid and gas phase in the product provide consistent pressures as long as there is liquid phase present—the pressure in the headspace is maintained by the equilibrium between the two phases. In contrast, the pressure provided by propellants that have no liquid phase may change relatively rapidly as the contents of the container are expelled. As the headspace becomes larger, the pressure within the container falls proportionately. Propellants that have no liquid phase but have significant pressure-dependent solubility in the rest of the formulation have performance characteristics between the other two systems. In such cases, as the headspace increases, the propellant comes out of solution to help to maintain the pressure of the system.
In metered-dose inhalers the propellant has a liquid phase that is an integral part of the dispensed pharmaceutical product. Actuating the metering valve dispenses a defined volume of the liquid contents. The propellant spontaneously boils and provides atomizing and propulsive force. A predictable change in active concentration occurs from the beginning to the end of the container life cycle as the liquid phase of the propellant vaporizes to reestablish the equilibrium pressure of the system as the headspace increases.
 Physical Properties:
Propellants have boiling points well below ambient temperatures. Density and solubility properties are significant considerations when one selects a propellant. Apaflurane and norflurane have liquid-phase densities that are greater than that of water. Hydrocarbon propellants (butane, isobutene, and propane) and dimethyl ether have liquid-phase densities that are less than that of water. Physical Properties:
Propellants have boiling points well below ambient temperatures. Density and solubility properties are significant considerations when one selects a propellant. Apaflurane and norflurane have liquid-phase densities that are greater than that of water. Hydrocarbon propellants (butane, isobutene, and propane) and dimethyl ether have liquid-phase densities that are less than that of water.
 Chemical Properties:
Propellants typically are stable materials that contribute to long shelf lives of formulations. However, the hydrocarbon propellants (butane, isobutene, and propane) and dimethyl ether are all flammable materials. Apaflurane, carbon dioxide, nitrogen, and norflurane are nonflammable. Nitrous oxide is not flammable but supports combustion. Chlorofluorocarbon propellants are considered to be ozone-depleting substances. Their use in foods, drugs, devices, or cosmetics is regulated by 21 CFR 2.125. Albuterol metered-dose inhalers formulated with chlorofluorocarbon propellants have not been available in the United States since January 1, 2009. Chemical Properties:
Propellants typically are stable materials that contribute to long shelf lives of formulations. However, the hydrocarbon propellants (butane, isobutene, and propane) and dimethyl ether are all flammable materials. Apaflurane, carbon dioxide, nitrogen, and norflurane are nonflammable. Nitrous oxide is not flammable but supports combustion. Chlorofluorocarbon propellants are considered to be ozone-depleting substances. Their use in foods, drugs, devices, or cosmetics is regulated by 21 CFR 2.125. Albuterol metered-dose inhalers formulated with chlorofluorocarbon propellants have not been available in the United States since January 1, 2009.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question |
Contact |
Expert Committee |
| General Chapter |
Robert H. Lafaver, M.S.
Scientific Liaison
1-301-816-8335 |
(GCPA2010) General Chapters - Physical Analysis |
USP35–NF30 Page 598
Pharmacopeial Forum: Volume No. 35(5) Page 1228
|
 846
846 , Crystallinity
, Crystallinity  695
695 , Chromatography
, Chromatography  621
621 , Water Determination
, Water Determination  921
921 , Melting Range or Temperature
, Melting Range or Temperature  741
741 , and Iron
, and Iron  241
241 .
.
 1231
1231 .
.