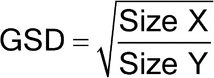This general chapter contains test methods for propellants, pressurized topical aerosols, nasal sprays, metered-dose inhalers, and propellant-free dry powder inhalers used to aerosolize, or to aerosolize and meter, doses of powders for inhalation. Apply these methods, where indicated, in the testing of the appropriate dosage forms.
PROPELLANTS
Caution—Hydrocarbon propellants are highly flammable and explosive. Observe precautions and perform sampling and analytical operations in a well-ventilated fume hood.
General Sampling Procedure
This procedure is used to obtain test specimens for those propellants that occur as gases at about 25 and that are stored in pressurized cylinders. Use a stainless steel sample cylinder equipped with a stainless steel valve and having a capacity of not less than 200 mL and a pressure rating of 240 psi or more. Dry the cylinder with the valve open at 110
and that are stored in pressurized cylinders. Use a stainless steel sample cylinder equipped with a stainless steel valve and having a capacity of not less than 200 mL and a pressure rating of 240 psi or more. Dry the cylinder with the valve open at 110 for 2 hours, and evacuate the hot cylinder to less than 1 mm of mercury. Close the valve, cool, and weigh. Connect one end of a charging line tightly to the propellant container and the other end loosely to the sample cylinder. Carefully open the propellant container, and allow the propellant to flush out the charging line through the loose connection. Avoid excessive flushing, which causes moisture to freeze in the charging line and connections. Tighten the fitting on the sample cylinder, and open the sample cylinder valve, allowing the propellant to flow into the evacuated cylinder. Continue sampling until the desired amount of specimen is obtained, then close the propellant container valve, and finally close the sample cylinder valve. [Caution—Do not overload the sample cylinder; hydraulic expansion due to temperature change can cause overloaded cylinders to explode.
] Again weigh the charged sample cylinder, and calculate the weight of the specimen.
for 2 hours, and evacuate the hot cylinder to less than 1 mm of mercury. Close the valve, cool, and weigh. Connect one end of a charging line tightly to the propellant container and the other end loosely to the sample cylinder. Carefully open the propellant container, and allow the propellant to flush out the charging line through the loose connection. Avoid excessive flushing, which causes moisture to freeze in the charging line and connections. Tighten the fitting on the sample cylinder, and open the sample cylinder valve, allowing the propellant to flow into the evacuated cylinder. Continue sampling until the desired amount of specimen is obtained, then close the propellant container valve, and finally close the sample cylinder valve. [Caution—Do not overload the sample cylinder; hydraulic expansion due to temperature change can cause overloaded cylinders to explode.
] Again weigh the charged sample cylinder, and calculate the weight of the specimen.
Approximate Boiling Temperature
Transfer a 100-mL specimen to a tared, pear-shaped, 100-mL centrifuge tube containing a few boiling stones, and weigh. Suspend a thermometer in the liquid, and place the tube in a medium maintained at a temperature of 32 above the expected boiling temperature. When the thermometer reading becomes constant, record as the boiling temperature the thermometer reading after at least 5% of the specimen has distilled. Retain the remainder of the specimen for the determination of High-Boiling Residues.
above the expected boiling temperature. When the thermometer reading becomes constant, record as the boiling temperature the thermometer reading after at least 5% of the specimen has distilled. Retain the remainder of the specimen for the determination of High-Boiling Residues.
High-Boiling Residues, Method I
Allow 85 mL of the specimen to distill as directed in the test for Approximate Boiling Temperature, and transfer the centrifuge tube containing the remaining 15 mL of specimen to a medium maintained at a temperature 10 above the boiling temperature. After 30 minutes, remove the tube from the water bath, blot dry, and weigh. Calculate the weight of the residue.
above the boiling temperature. After 30 minutes, remove the tube from the water bath, blot dry, and weigh. Calculate the weight of the residue.
High-Boiling Residues, Method II
Prepare a cooling coil from copper tubing (about 6 mm outside diameter × about 6.1 m long) to fit into a vacuum-jacketed flask. Immerse the cooling coil in a mixture of dry ice and acetone in a vacuum-jacketed flask, and connect one end of the tubing to the propellant sample cylinder. Carefully open the sample cylinder valve, flush the cooling coil with about 50 mL of the propellant, and discard this portion of liquefied propellant. Continue delivering liquefied propellant from the cooling coil, and collect it in a previously chilled 1000-mL sedimentation cone until the cone is filled to the 1000-mL mark. Allow the propellant to evaporate, using a warm water bath maintained at about 40 to reduce evaporating time. When all of the liquid has evaporated, rinse the sedimentation cone with two 50-mL portions of pentane, and combine the rinsings in a tared 150-mL evaporating dish. Transfer 100 mL of the pentane solvent to a second tared 150-mL evaporating dish, place both evaporating dishes on a water bath, evaporate to dryness, and heat the dishes in an oven at 100
to reduce evaporating time. When all of the liquid has evaporated, rinse the sedimentation cone with two 50-mL portions of pentane, and combine the rinsings in a tared 150-mL evaporating dish. Transfer 100 mL of the pentane solvent to a second tared 150-mL evaporating dish, place both evaporating dishes on a water bath, evaporate to dryness, and heat the dishes in an oven at 100 for 60 minutes. Cool the dishes in a desiccator, and weigh. Repeat the heating for 15-minute periods until successive weighings are within 0.1 mg, and calculate the weight of the residue obtained from the propellant as the difference between the weights of the residues in the two evaporating dishes.
for 60 minutes. Cool the dishes in a desiccator, and weigh. Repeat the heating for 15-minute periods until successive weighings are within 0.1 mg, and calculate the weight of the residue obtained from the propellant as the difference between the weights of the residues in the two evaporating dishes.
Water Content
Proceed as directed under Water Determination  921
921 , with the following modifications: (a) Provide the closed-system titrating vessel with an opening through which passes a coarse-porosity gas dispersion tube connected to a sampling cylinder. (b) Dilute the Reagent with anhydrous methanol to give a water equivalence factor of between 0.2 and 1.0 mg per mL; age this diluted solution for not less than 16 hours before standardization. (c) Obtain a 100-g specimen as directed under General Sampling Procedure, and introduce the specimen into the titration vessel through the gas dispersion tube at a rate of about 100 mL of gas per minute; if necessary, heat the sample cylinder gently to maintain this flow rate.
, with the following modifications: (a) Provide the closed-system titrating vessel with an opening through which passes a coarse-porosity gas dispersion tube connected to a sampling cylinder. (b) Dilute the Reagent with anhydrous methanol to give a water equivalence factor of between 0.2 and 1.0 mg per mL; age this diluted solution for not less than 16 hours before standardization. (c) Obtain a 100-g specimen as directed under General Sampling Procedure, and introduce the specimen into the titration vessel through the gas dispersion tube at a rate of about 100 mL of gas per minute; if necessary, heat the sample cylinder gently to maintain this flow rate.
Other Determinations
For those aerosols that use propellants, perform the tests specified in the individual NF propellant monographs.
AEROSOLS
Because leaching of extractable substances into pressurized formulations should be minimized, valve materials and other components that are in contact with the product meet the requirements under Elastomeric Closures for Injections  381
381 (Note that under Physicochemical Test Procedures in
(Note that under Physicochemical Test Procedures in  381
381 the directions for preparing a sample require pre-extraction, which may cause an underestimate of the amount of extractables from a given component.) See also Aerosols under Pharmaceutical Dosage Forms
the directions for preparing a sample require pre-extraction, which may cause an underestimate of the amount of extractables from a given component.) See also Aerosols under Pharmaceutical Dosage Forms  1151
1151 .
.
TOPICAL AEROSOLS
The following tests are applicable to topical aerosols containing drug, in suspension or solution, packaged under pressure, and released upon activation of an appropriate valve system.
Delivery Rate and Delivered Amount
Perform these tests only on containers fitted with continuous valves.
Delivery Rate—
Select not fewer than four aerosol containers, shake, if the label includes this directive, remove the caps and covers, and actuate each valve for 2 to 3 seconds. Weigh each container accurately, and immerse in a constant-temperature bath until the internal pressure is equilibrated at a temperature of 25 as determined by constancy of internal pressure, as directed under the Pressure Test below. Remove the containers from the bath, remove excess moisture by blotting with a paper towel, shake, if the label includes this directive, actuate each valve for 5.0 seconds (accurately timed by use of a stopwatch), and weigh each container again. Return the containers to the constant-temperature bath, and repeat the foregoing procedure three times for each container. Calculate the average Delivery Rate, in g per second, for each container.
as determined by constancy of internal pressure, as directed under the Pressure Test below. Remove the containers from the bath, remove excess moisture by blotting with a paper towel, shake, if the label includes this directive, actuate each valve for 5.0 seconds (accurately timed by use of a stopwatch), and weigh each container again. Return the containers to the constant-temperature bath, and repeat the foregoing procedure three times for each container. Calculate the average Delivery Rate, in g per second, for each container.
Delivered Amount—
Return the containers to the constant-temperature bath, continuing to deliver 5 second actuations to waste, until each container is exhausted. [Note—Ensure that sufficient time is allowed between each actuation to avoid significant canister cooling. ] Calculate the total weight loss from each container. This is the Delivered Amount.
Pressure Test
Perform this test only on topical aerosols fitted with continuous valves.
Select not fewer than four aerosol containers, remove the caps and covers, and immerse in a constant-temperature bath until the internal pressure is constant at a temperature of 25 . Remove the containers from the bath, shake, and remove the actuator and water, if any, from the valve stem. Place each container in an upright position, and determine the pressure in each container by placing a calibrated pressure gauge on the valve stem, holding firmly, and actuating the valve so that it is fully open. The gauge is of a calibration approximating the expected pressure and is fitted with an adapter appropriate for the particular valve stem dimensions. Read the pressure directly from the gauge.
. Remove the containers from the bath, shake, and remove the actuator and water, if any, from the valve stem. Place each container in an upright position, and determine the pressure in each container by placing a calibrated pressure gauge on the valve stem, holding firmly, and actuating the valve so that it is fully open. The gauge is of a calibration approximating the expected pressure and is fitted with an adapter appropriate for the particular valve stem dimensions. Read the pressure directly from the gauge.
Minimum Fill
Topical aerosols meet the requirements for aerosols under Minimum Fill  755
755 .
.
Leakage Test
Perform this test only on topical aerosols fitted with continuous valves.
Select 12 aerosol containers, and record the date and time to the nearest half hour. Weigh each container to the nearest mg, and record the weight, in mg, of each as W1. Allow the containers to stand in an upright position at a temperature of 25.0 ± 2.0 for not less than 3 days, and again weigh each container, recording the weight, in mg, of each as W2, and recording the date and time to the nearest half hour. Determine the time, T, in hours, during which the containers were under test. Calculate the leakage rate, in mg per year, of each container taken by the formula:
for not less than 3 days, and again weigh each container, recording the weight, in mg, of each as W2, and recording the date and time to the nearest half hour. Determine the time, T, in hours, during which the containers were under test. Calculate the leakage rate, in mg per year, of each container taken by the formula:
(365)(24/T)(W1  W2).
W2).
Where plastic-coated glass aerosol containers are tested, dry the containers in a desiccator for 12 to 18 hours, and allow them to stand in a constant-humidity environment for 24 hours prior to determining the initial weight as indicated above. Conduct the test under the same constant-humidity conditions. Empty the contents of each container tested by employing any safe technique (e.g., chill to reduce the internal pressure, remove the valve, and pour). Remove any residual contents by rinsing with suitable solvents, then rinse with a few portions of methanol. Retain as a unit the container, the valve, and all associated parts, and heat them at 100 for 5 minutes. Cool, weigh, record the weight as W3, and determine the net fill weight (W1
for 5 minutes. Cool, weigh, record the weight as W3, and determine the net fill weight (W1  W3) for each container tested. [Note—If the average net fill weight has been determined previously, that value may be used in place of the value (W1
W3) for each container tested. [Note—If the average net fill weight has been determined previously, that value may be used in place of the value (W1  W3) above. ] The requirements are met if the average leakage rate per year for the 12 containers is not more than 3.5% of the net fill weight, and none of the containers leaks more than 5.0% of the net fill weight per year. If 1 container leaks more than 5.0% per year, and if none of the containers leaks more than 7.0% per year, determine the leakage rate of an additional 24 containers as directed herein. Not more than 2 of the 36 containers leak more than 5.0% of the net fill weight per year, and none of the 36 containers leaks more than 7.0% of the net fill weight per year. Where the net fill weight is less than 15 g and the label bears an expiration date, the requirements are met if the average leakage rate of the 12 containers is not more than 525 mg per year and none of the containers leaks more than 750 mg per year. If 1 container leaks more than 750 mg per year, but not more than 1.1 g per year, determine the leakage rate of an additional 24 containers as directed herein. Not more than 2 of the 36 containers leak more than 750 mg per year, and none of the 36 containers leaks more than 1.1 g per year. This test is in addition to the customary in-line leak testing of each container.
W3) above. ] The requirements are met if the average leakage rate per year for the 12 containers is not more than 3.5% of the net fill weight, and none of the containers leaks more than 5.0% of the net fill weight per year. If 1 container leaks more than 5.0% per year, and if none of the containers leaks more than 7.0% per year, determine the leakage rate of an additional 24 containers as directed herein. Not more than 2 of the 36 containers leak more than 5.0% of the net fill weight per year, and none of the 36 containers leaks more than 7.0% of the net fill weight per year. Where the net fill weight is less than 15 g and the label bears an expiration date, the requirements are met if the average leakage rate of the 12 containers is not more than 525 mg per year and none of the containers leaks more than 750 mg per year. If 1 container leaks more than 750 mg per year, but not more than 1.1 g per year, determine the leakage rate of an additional 24 containers as directed herein. Not more than 2 of the 36 containers leak more than 750 mg per year, and none of the 36 containers leaks more than 1.1 g per year. This test is in addition to the customary in-line leak testing of each container.
Number of Discharges per Container
Perform this test only on topical aerosols fitted with dose-metering valves, at the same time as, and on the same containers used for, the test for Delivered-Dose Uniformity. Determine the number of discharges or deliveries by counting the number of priming discharges plus those used in determining the spray contents, and continue to fire until the label claim number of discharges. The requirements are met if all the containers or inhalers tested contain not less than the number of discharges stated on the label.
Delivered-Dose Uniformity
The test for Delivered-Dose Uniformity is required for topical aerosols fitted with dose-metering valves. For collection of the minimum dose, proceed as directed in the test for Delivered-Dose Uniformity under Metered-Dose Inhalers and Dry Powder Inhalers, as described below, except to modify the dose sampling apparatus so that it is capable of quantitatively capturing the delivered dose from the preparation being tested. Unless otherwise stated in the individual monograph, apply the acceptance criteria for Metered-Dose Inhalers and Dry Powder Inhalers as described below.
NASAL SPRAYS
The following test is applicable to nasal sprays, formulated as aqueous suspensions or solutions of drug, presented in multi-dose containers and fitted with dose-metering valves. In all cases, and for all tests, prepare and test the nasal spray as directed on the label and the instructions for use.
Delivered-Dose Uniformity
Unless otherwise directed in the individual monograph, the drug content of the minimum delivered doses (minimum number of sprays per nostril as described on the label, or instructions for use) collected at the beginning of unit life (after priming as described on the label, or instructions for use) and at the label claim number of metered sprays, from each of 10 separate containers, must meet the following acceptance criteria: not more than 2 of the 20 doses are outside the range of 80% to 120% of label claim, and none are outside the range of 75% to 125% of label claim, while the mean for each of the beginning and end doses falls within the range of 85% to 115% of label claim. If 3–6 doses of the 20 doses collected are outside of 80% to 120% of the label claim, but none are outside of 75% to 125% of label claim, and the means for each of the beginning and end doses fall within 85% to 115% of label claim, select 20 additional containers for second-tier testing. For second-tier testing, the requirements are met if not more than 6 of the 60 doses collected are outside the range of 80% to 120% of label claim, none are outside the range of 75% to 125% of label claim, and the means for each of the beginning and end doses fall within the range of 85% to 115% of label claim.
sampling for delivered-dose uniformity of metered-dose nasal sprays
General Sampling Procedure—
To ensure reproducible in-vitro dose collection, it is recommended that a mechanical means of actuating the pump assembly be employed to deliver doses for collection. The mechanical actuation procedure should have adequate controls for the critical mechanical actuation parameters (e.g., actuation force, actuation speed, stroke length, rest periods, etc.). The test must be performed on units that have been primed according to the patient-use instructions. The test unit should be actuated in a vertical or near vertical, valve-up, position. The two doses collected at the beginning and end of the container life should be the dose immediately following priming and the dose corresponding to the last label claim number of doses from the container.
For suspension products, the delivered dose should be delivered into a suitable container (e.g., scintillation vial) in which quantitative transfer from the container under test can be accomplished. A validated analytical method is employed to determine the amount of drug in each delivered dose, and data are reported as a percent of label claim. For solution products, the delivered dose can be determined gravimetrically from the weight of the delivered dose, and the concentration and density of the fill solution of the product under test.
METERED-DOSE INHALERS AND DRY POWDER INHALERS
The following tests are applicable to metered-dose inhalers that are formulated as suspensions or solutions of active drug in propellants and dry powder inhalers presented as single or multidose units. The following test methods are specific to the aforementioned inhalers and may require modification when testing alternative inhalation technologies (for example, breath-actuated metered-dose inhalers, or dose-metering nebulizers). However, Pharmacopeial requirements for all dose-metering inhalation dosage forms require determination of the delivered dose and Aerodynamic Size Distribution. In all cases, and for all tests, prepare and test the inhaler as directed on the label and the instructions for use. When these directions are not provided by the product manufacturer, follow the precise dose discharge directions included in the tests below.
Delivered-Dose Uniformity
The test for Delivered-Dose Uniformity is required for inhalers (e.g., metered-dose inhalers or dry powder inhalers) containing drug formulation (e.g., solution, suspension, or powder) either in reservoirs or in premetered dosage units, and for drug formulations packaged in reservoirs or in premetered dosage units where these containers are labeled for use with a named inhalation device. (For inhalations packaged in premetered dosage units, see also Uniformity of Dosage Units  905
905 .) Note that the target-delivered dose is the expected mean drug content for a large number of delivered doses collected from many inhalers of the chosen product. In many cases, its value may depend upon the manner in which the test for delivered dose is performed. For metered-dose inhalers, the target-delivered dose is specified by the label claim, unless otherwise specified in the individual monograph. For dry powder inhalers, where the label claim is usually the packaged or metered-dose of drug, the target-delivered dose is specified in the individual monograph and is usually less than the label claim. Its value reflects the expected mean drug content for a large number of delivered doses collected from the product, using the method specified in the monograph.
.) Note that the target-delivered dose is the expected mean drug content for a large number of delivered doses collected from many inhalers of the chosen product. In many cases, its value may depend upon the manner in which the test for delivered dose is performed. For metered-dose inhalers, the target-delivered dose is specified by the label claim, unless otherwise specified in the individual monograph. For dry powder inhalers, where the label claim is usually the packaged or metered-dose of drug, the target-delivered dose is specified in the individual monograph and is usually less than the label claim. Its value reflects the expected mean drug content for a large number of delivered doses collected from the product, using the method specified in the monograph.
Unless otherwise directed in the individual monograph, the drug content of the minimum delivered dose from each of 10 separate containers is determined in accordance with the procedure described below.
Unless otherwise specified in the individual monograph, the requirements for dosage uniformity are met if not less than 9 of the 10 doses are between 75% and 125% of the specified target-delivered dose and none is outside the range of 65% to 135% of the specified target-delivered dose. If the contents of not more than 3 doses are outside the range of 75% to 125% of the specified target-delivered dose, but within the range of 65% to 135% of the specified target-delivered dose, select 20 additional containers, and follow the prescribed procedure for analyzing 1 minimum dose from each. The requirements are met if not more than 3 results, out of the 30 values, lie outside the range of 75% to 125% of the specified target-delivered dose, and none is outside the range of 65% to 135% of the specified target-delivered dose.
sampling the delivered dose from metered-dose inhalers
To determine the content of active ingredient in the discharged spray from a metered-dose inhaler, use the sampling apparatus described below, using a flow rate of 28.3 L of air per minute (±5%), unless otherwise stated in the individual monograph.
Apparatus A—
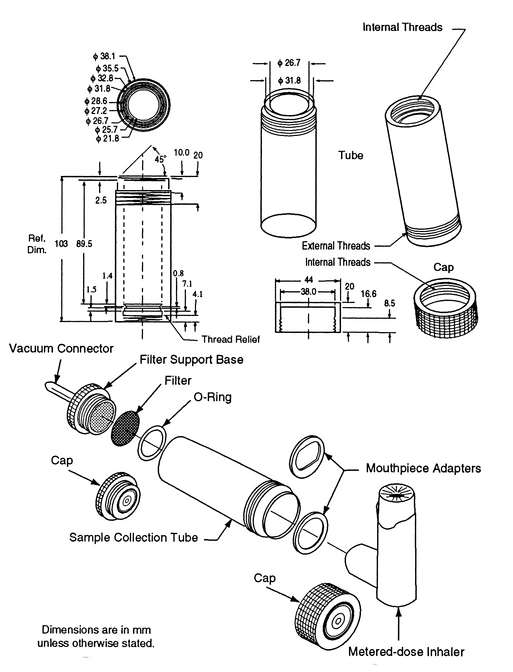
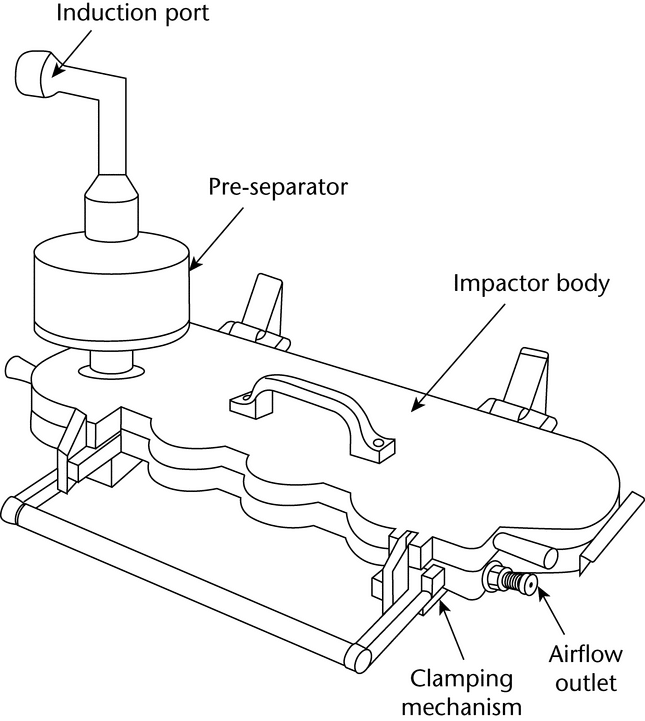
The apparatus (see Figure 1)
consists of a filter support base with an open-mesh filter support, such as a stainless steel screen, a collection tube that is clamped or screwed to the filter support base, and a mouthpiece adapter to ensure an airtight seal between the collection tube and the mouthpiece. Use a mouthpiece adapter that ensures that the opening of the inhaler mouthpiece is flush with the front face or 2.5-mm indented shoulder in the sample collection tube, as appropriate. The vacuum connector is connected to a system comprising a vacuum source, flow regulator, and flowmeter. The source should be capable of pulling air through the complete assembly, including the filter and the inhaler to be tested, at the desired flow rate. When testing metered-dose inhalers, air should be drawn continuously through the system to avoid loss of drug into the atmosphere. The filter support base is designed to accommodate 25-mm diameter filter disks. At the airflow being used, the sample collection tube and the filter disk must be capable of quantitatively collecting the Delivered Dose. The filter disk and other materials used in the construction of the apparatus must be compatible with the drug and the solvents that are used to extract the drug from the filter. One end of the collection tube is designed to hold the filter disk tightly against the filter support base. When assembled, the joints between the components of the apparatus are airtight so that when a vacuum is applied to the base of the filter, all of the air drawn through the collection device passes through the inhaler.
Procedure—
Prepare the inhaler for use according to the label instructions. Unless otherwise specified in the individual monograph, with the vacuum pump running, ensuring an airflow rate through the inhaler of 28.3 L of air per minute (±5%), discharge the minimum recommended dose into the apparatus through the mouthpiece adapter by depressing the valve for a duration sufficient to ensure that the dose has been completely discharged. Detach the inhaler from Apparatus A, and disconnect the vacuum. Assay the contents of the apparatus for drug after rinsing the filter and the interior of the apparatus with a suitable solvent.
sampling the delivered dose from dry powder inhalers
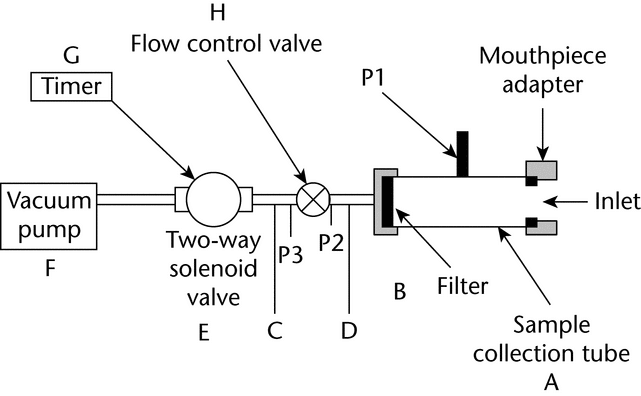
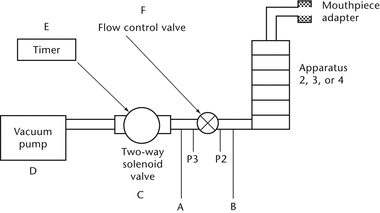
To determine the content of active ingredient emitted from the mouthpiece of a dry powder inhaler, use Apparatus B (see Figure 2).
Fig. 2. Apparatus B: Sampling apparatus for dry powder inhalers. (See Table 1 for component specifications.)
Apparatus B: Sampling apparatus for dry powder inhalers. (See Table 1 for component specifications.)
Table 1. Component Specifications for Apparatus B (see Fig. 2)
| Code | Item | Description | Dimensions |
|---|---|---|---|
| A | Sample collection tubea | See Fig. 2 | 34.85-mm ID × 12-cm length |
| B | Filterb | See Fig. 2 | 47-mm glass fiber filter |
| C | Connector | (e.g., short metal coupling with low diameter branch to P3) | |
| D | Vacuum tubing | (e.g., silicon tubing with an outside diameter of 14 mm and an internal diameter of 8 mm) | A length of suitable tubing |
| E | Two-way solenoid valvec | See Fig. 2 | 2-way, 2-port solenoid valve having an ID |
| F | Vacuum pumpd | See Fig. 2 | Pump must be capable of drawing the required flow rate through the assembled apparatus with the dry powder inhaler in the mouthpiece adapter. Connect the pump to the solenoid valve using short and wide ( |
| G | Timere | See Fig. 2 | The timer switches current directly to the solenoid valve for the required duration. |
| P1 | Pressure tap | See Fig. 2 | 2.2-mm ID, 3.1-mm OD flush with the internal surface of the sample collection tube, centered and burr free, 59 mm from its inlet. The pressure taps P1, P2, and P3 must not be open to the atmosphere during dose collection. |
| P1, P2, P3 | Pressure measurementsf | ||
| H | Flow-control valveg | See Fig. 2 | Adjustable regulating valve with maximum Cv |
|
a
An example being a Millipore product number XX40 047 00 (Millipore Corporation, 80, Ashby Road, Bedford, MA 01732), modified so that the exit tube has an ID
b
A/E (Gelman Sciences Inc., 600 South Wagner Road, Ann Arbor, MI 48106) or equivalent.
c
ASCO product number 8030G13, Automatic Switch Company, 60 Hanover Road, Florham Park, NJ 07932.
d
Gast product type 1023, 1423, or 2565 (Gast Manufacturing Inc., PO Box 97, Benton Harbor, MI 49022) or equivalent.
e
Eaton Product number 45610-400 (Eaton Corporation, Automotive Products Division, 901, South 12th Street, Watertown, WI 53094) or equivalent.
f
An example being a PDM 210 pressure meter (Air-Neotronics Ltd., Neotronics Technology plc, Parsonage Road, Takeley, Bishop's Stortford, CM22 6PU, UK), or equivalent.
g
Parker Hannifin type 8FV12LNSS (Parker Hannifin plc., Riverside Road, Barnstable, Devon EX31 1NP, UK) or equivalent.
h
Flow Coefficient, as defined by ISA S75.02 “Control valve capacity test procedure” in Standards and Recommended Practices for Instrumentation and Control, 10th ed., Vol. 2, 1989. Published by Instrument Society of America, 67 Alexander Drive, P.O. Box 1227, Research Triangle Park, NC 27709, U.S.A.
|
This apparatus is capable of sampling the emitted doses at a variety of airflow rates.
Apparatus B—
The apparatus is similar to that described in Figure 1 for testing metered-dose inhalers. In this case, however, the filter and collection tube have a larger internal diameter to accommodate 47-mm diameter filter disks. This feature enables dosage collection at higher airflow rates—up to 100 L of air per minute—when necessary. A mouthpiece adapter ensures an airtight seal between the collection tube and the mouthpiece of the dry powder inhaler being tested. The mouthpiece adapter must ensure that the tip of the inhaler mouthpiece is flush with the open end of the sample collection tube. Tubing connectors, if they are used, should have an internal diameter greater than or equal to 8 mm to preclude their own internal diameters from creating significant airflow resistance. A vacuum pump with excess capacity must be selected in order to draw air, at the designated volumetric flow rate, through both the sampling apparatus and the inhaler simultaneously. A timer-controlled, low resistance, solenoid-operated, two-way valve is interposed between the vacuum pump and the flow-control valve to control the duration of flow. This type of valve enables 4.0 L of air (±5%) to be withdrawn from the mouthpiece of the inhaler at the designated flow rate. Flow control is achieved by ensuring that critical (sonic) flow occurs in the flow-control valve (absolute pressure ratio P3/P2 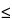 0.5 under conditions of steady-state flow).
0.5 under conditions of steady-state flow).
Procedure—
Operate the apparatus at an airflow rate that produces a pressure drop of 4 kPa (40.8 cm H2O) over the inhaler to be tested and at a duration consistent with the withdrawal of 4 L of air from the mouthpiece of the inhaler. [Note—If the flow rate and duration are defined otherwise in the monograph, adjust the system to within 5% of those values. ] Determine the test flow rate using Apparatus B as follows. Insert an inhaler into the mouthpiece adapter to ensure an airtight seal. In cases where the drug packaging modifies the inhaler's resistance to airflow, use a loaded, drug-free inhaler (with previously emptied packaging). In other cases, use an unloaded (drug free) inhaler. Connect one port of a differential pressure transducer to the pressure tap, P1, and leave the other open to the atmosphere. Switch on the pump, and open the two-way solenoid valve. Adjust the flow-control valve until the pressure drop across the inhaler is 4.0 kPa (40.8 cm H2O). Ensure that critical (sonic) flow occurs in the flow-control valve by determining the individual values for absolute pressure, P2 and P3, so that their ratio P3/P2 is less than or equal to 0.5. If this criterion cannot be achieved, it is likely that the vacuum pump is worn or of insufficient capacity. Critical (sonic) flow conditions in the flow-control valve are required in order to ensure that the volumetric airflow drawn from the mouthpiece is unaffected by pump fluctuations and changes in airflow resistance of the inhaler. Remove the inhaler from the mouthpiece adapter and without disturbing the flow-control valve, measure the airflow rate drawn from the mouthpiece, Qout, by connecting a flowmeter to the mouthpiece adaptor in an airtight fashion. Use a flowmeter calibrated for the volumetric flow leaving the meter in an airtight fashion to directly determine Qout or, if such a meter is unobtainable, calculate the volumetric flow leaving the meter (Qout) using the ideal gas law. For example, for a meter calibrated for the entering volumetric flow (Qin), use the formula:
 1 times beginning with the text, “Prime or load the inhaler with powder,” where n is the number of times defined in the labeling as the minimum recommended dose. Detach the dry powder inhaler from the sampling apparatus, and disconnect the vacuum tubing, D. Assay the contents of the apparatus for drug after rinsing the filter and the interior of the apparatus with a suitable solvent. Where specified in individual monographs, perform this test under conditions of controlled temperature and humidity.
1 times beginning with the text, “Prime or load the inhaler with powder,” where n is the number of times defined in the labeling as the minimum recommended dose. Detach the dry powder inhaler from the sampling apparatus, and disconnect the vacuum tubing, D. Assay the contents of the apparatus for drug after rinsing the filter and the interior of the apparatus with a suitable solvent. Where specified in individual monographs, perform this test under conditions of controlled temperature and humidity.
Qout = QinP0 / (P0 – DP)
where P0 is the atmospheric pressure and DP is the pressure drop over the meter. If the flow rate is greater than 100 L of air per minute, adjust the flow-control valve until Qout equals 100 L per minute; otherwise, record the value of Qout, and leave the flow-control valve undisturbed. Define the test flow duration, T = 240/Qout, in seconds, so that a volume of 4.0 L of air (±5%) is withdrawn from the inhaler at the test flow rate Qout, and adjust the timer controlling the operation of the two-way solenoid valve accordingly. Prime or load the inhaler with powder for inhalation according to the labeled instructions. With the vacuum pump running and the solenoid valve closed, insert the inhaler mouthpiece horizontally into the mouthpiece adapter. Discharge the powder into the sampling apparatus by activating the timer controlling the solenoid valve and withdrawing 4.0 L of air from the inhaler at the previously defined airflow rate. If the labeled instructions so direct, repeat the operation so as to simulate the use of the inhaler by the patient (e.g., inhale two or three times, if necessary, to empty the capsule). Repeat the whole operation n
Delivered-Dose Uniformity over the Entire Contents
The test for Delivered-Dose Uniformity over the Entire Contents is required for inhalers (e.g., metered-dose inhalers or dry powder inhalers) containing multiple doses of drug formulation (e.g., solution, suspension, or dry powder) either in reservoirs or in premetered dosage units (e.g., blisters), and for drug formulations packaged in reservoirs or in multiple-dose assemblies of premetered dosage units that have a predetermined dose sequence, where these multiple-dose assemblies are labeled for use with a named inhalation device. The test for Delivered-Dose Uniformity over the Entire Contents also ensures that multidose products supply the total number of discharges stated on the label. Unless otherwise directed in the individual monograph, the drug content of at least 9 of the 10 doses collected from one inhaler, in accordance with the procedure below, are between 75% and 125% of the target-delivered dose, and none is outside the range of 65% to 135% of the target-delivered dose. If the contents of not more than 3 doses are outside the range of 75% to 125%, but within the range of 65% to 135% of the target-delivered dose, select 2 additional inhalers, and follow the prescribed procedure for analyzing 10 doses from each. The requirements are met if not more than 3 results, out of the 30 values, lie outside the range of 75% to 125% of the target-delivered dose, and none is outside the range of 65% to 135% of the target-delivered dose.
metered-dose inhalers
Apparatus—
Use Apparatus A as directed in Sampling the Delivered-Dose from Metered-Dose Inhalers under Delivered-Dose Uniformity at a flow rate of 28.3 L of air per minute (±5%).
Procedure—
A single dose is defined as the number of sprays specified in the product labeling as the minimum recommended dose. Select a single metered-dose inhaler, and follow the labeled instructions for priming, shaking, cleaning, and firing the inhaler throughout. Unless otherwise prescribed in the patient instructions, shake the inhaler for 5 seconds, and fire one minimum recommended dose to waste. Wait for 5 seconds, and collect the next dose. Detach the inhaler from Apparatus A, and disconnect the vacuum. Assay the contents of the apparatus for drug after rinsing the filter and the interior of the apparatus with a suitable solvent. Collect two more doses, allowing at least 5 seconds between doses. Discharge the device to waste, waiting for not less than 5 seconds between actuations (unless otherwise specified in the individual monograph), until (n/2) + 1 minimum recommended doses remain, in which n is the number of minimum recommended doses on the label. Collect four more doses, allowing at least 5 seconds between doses, unless otherwise specified in the individual monograph. Discharge the device to waste, as before, until three doses remain. Collect the final three doses, allowing at least 5 seconds between doses. Note that the rate of discharges to waste should not be such to cause excessive canister cooling.
dry powder inhalers
Apparatus—
Use Apparatus B as directed in Sampling the Delivered Dose from Dry Powder Inhalers under Delivered-Dose Uniformity at the appropriate airflow rate for testing.
Procedure—
Proceed as directed for Procedure in Sampling the Delivered Dose from Dry Powder Inhalers under Delivered-Dose Uniformity. A single dose is defined as the number of actuations stated in the product labeling as the minimum recommended dose. Select a single inhaler and follow the labeled instructions for loading with powder, discharging and cleaning throughout. Collect a total of 10 doses—three doses at the beginning, four in the middle [(n/2)  1 to (n/2) + 2, where n is the number of minimum recommended doses on the label], and three at the end—of the labeled contents following the labeled instructions. Prior to collecting each of the doses to be analyzed, clean the inhaler as directed in the labeling.
1 to (n/2) + 2, where n is the number of minimum recommended doses on the label], and three at the end—of the labeled contents following the labeled instructions. Prior to collecting each of the doses to be analyzed, clean the inhaler as directed in the labeling.
Particle Size
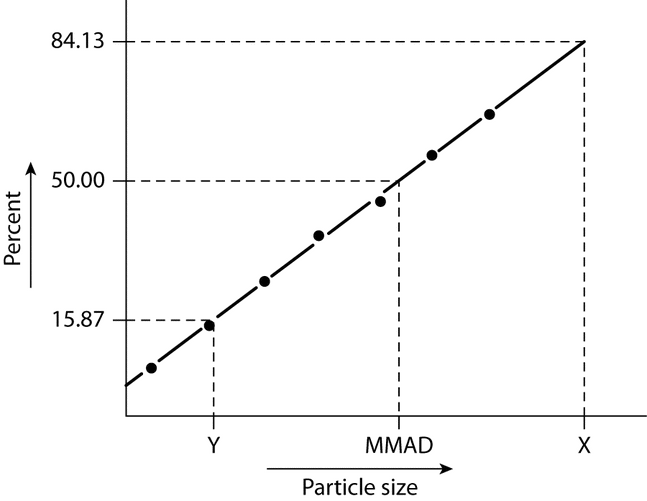
The particle or droplet size distribution in the spray discharged from metered-dose inhalers, and the particle size distribution in the cloud discharged from dry powder inhalers, are important characteristics used in judging inhaler performance. While particle size measurement by microscopy can be used to evaluate the number of large particles, agglomerates, and foreign particulates in the emissions of metered-dose inhalers (e.g., Epinephrine Bitartrate Inhalation Aerosol), whenever possible this test should be replaced with a method to determine the aerodynamic size distribution of the drug aerosol leaving the inhaler. The aerodynamic size distribution defines the manner in which an aerosol deposits during inhalation. When there is a log-normal distribution, the aerodynamic size distribution may be characterized by the mass median aerodynamic diameter (MMAD) and geometric standard deviation (GSD). The aerodynamic size distribution of the drug leaving metered-dose and dry powder inhalers is determined using Apparatus 1, 2, 3, 4, 5, or 6 as specified in this chapter. A fine particle dose or fine particle fraction can also be determined as that portion of the inhaler output having an aerodynamic diameter less than the size defined in the individual monograph. This may be expected to correlate with the drug dose or that fraction of the drug dose that penetrates the lung during inhalation. Individual monographs may also define the emitted fractions of the delivered dose in more than one aerodynamic size range.
aerodynamic size distribution
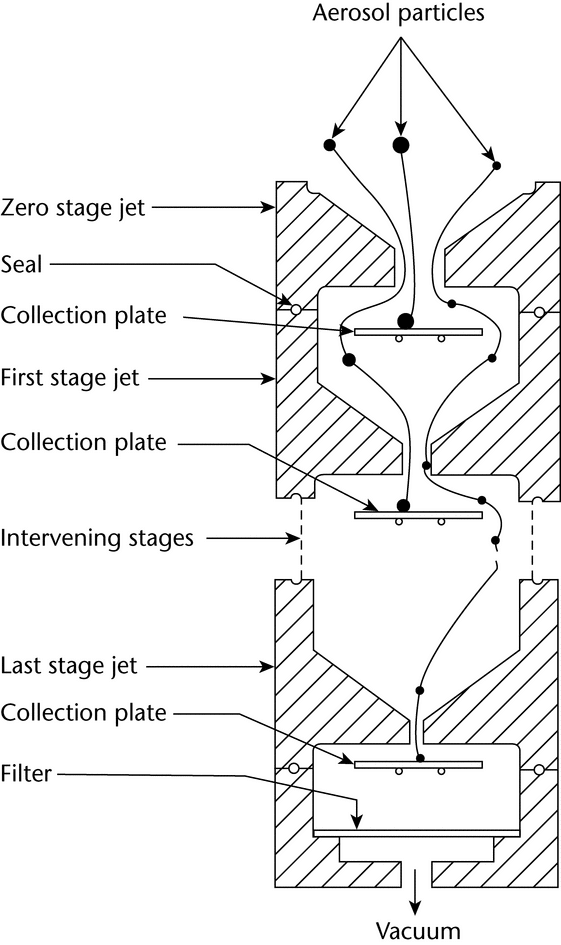
Cascade impaction devices classify aerosol particles and droplets on the basis of those particles' aerodynamic diameters. The principle of their operation, whereby they separate aerosol particles and droplets from a moving airstream on the basis of particle or droplet inertia, is shown in Figure 3.
Fig. 3. Schematic representation of the principle of operation of cascade impactors. (A single jet per impactor stage is shown. Impactors with multiple jets in each stage function in the same manner.)
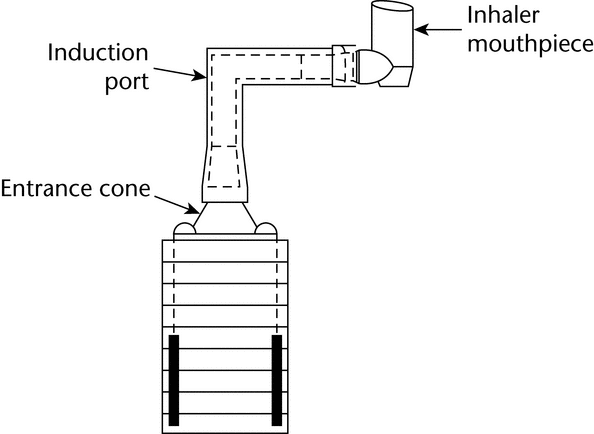
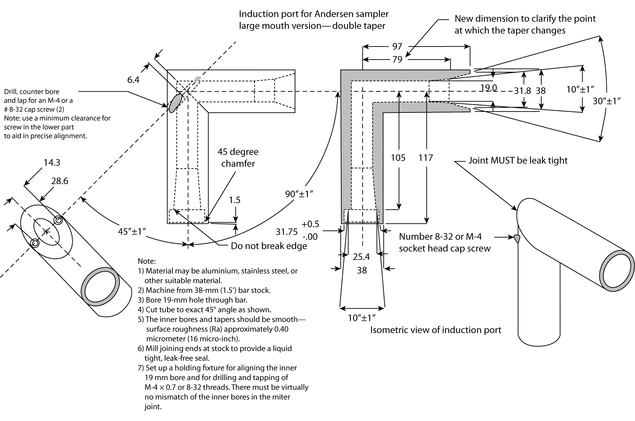
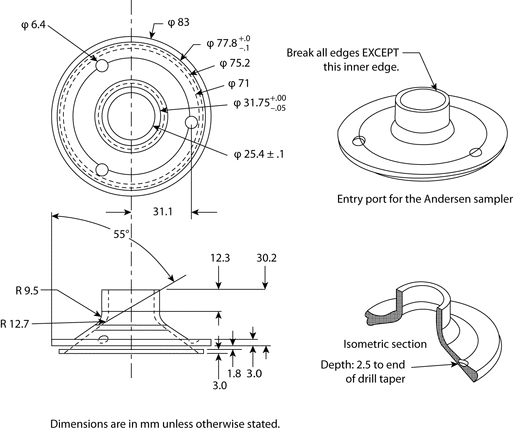
Because the dimensions of the induction port used to connect inhalers to the cascade impactors and impingers (shown in Apparatus 1, 2, 3, 4, 5, and 6) also define the mass of drug that enters the aerodynamic sizing device, these are carefully defined and, where possible, are held constant between each apparatus (see Figures 4, 6, 7, 8, and 9).
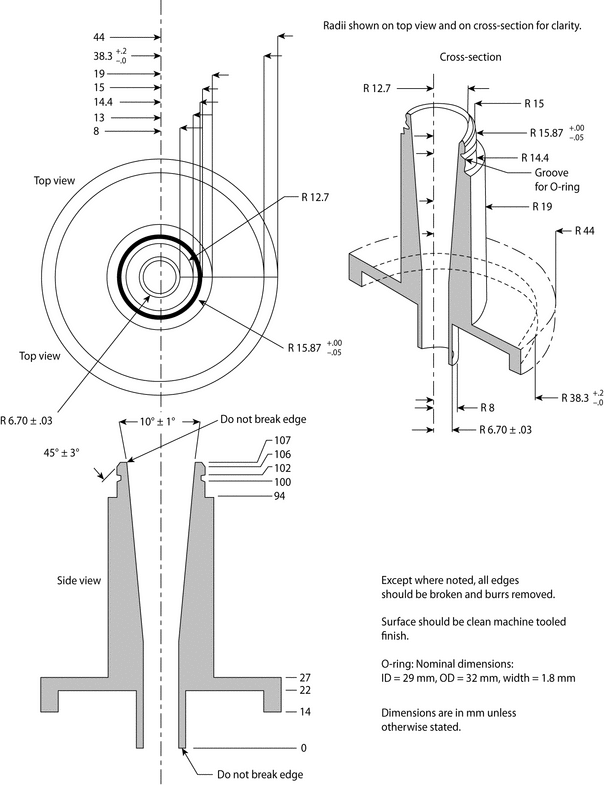
Fig. 4a. Apparatus 1: Expanded view of induction port for use with metered-dose and dry powder inhalers.
Fig. 4b. Apparatus 1: Expanded view of the entrance cone for mounting induction port on the Andersen cascade impactor without preseparator. Material may be aluminum, stainless steel, or other suitable material. Surface roughness (Ra) should be approximately 0.4 µm.
Fig. 5. Apparatus 2, 3, 4, or 5: General control equipment. (See Table 3 for component specifications.)
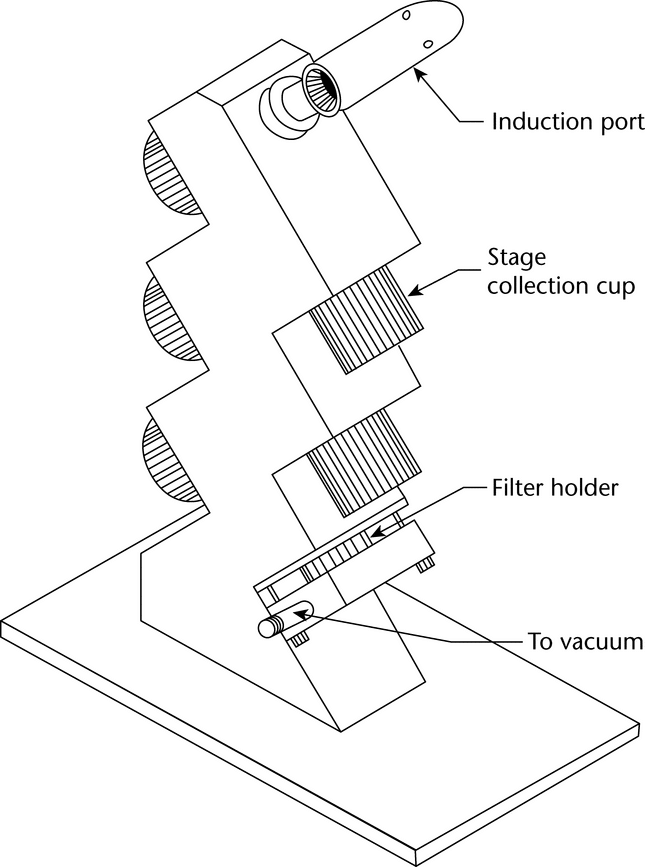
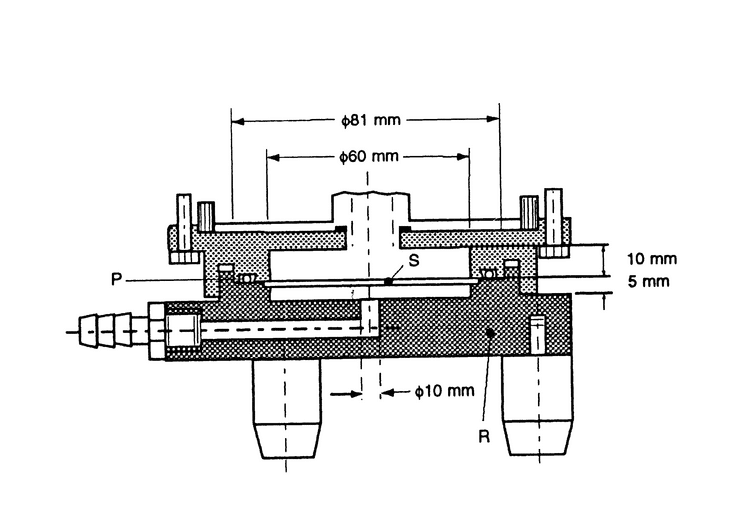
Fig. 6. Apparatus 2: Assembly of induction port, stage collector, and filter holder. (Marple-Miller impactor, Model 160 with USP induction port.)
Fig. 7. Apparatus 3: Expanded views of top for the Andersen preseparator adapted to the USP induction port. Material may be aluminum, stainless steel, or other suitable material; interior bore should be polished to surface roughness (Ra) approximately 0.4 µm.
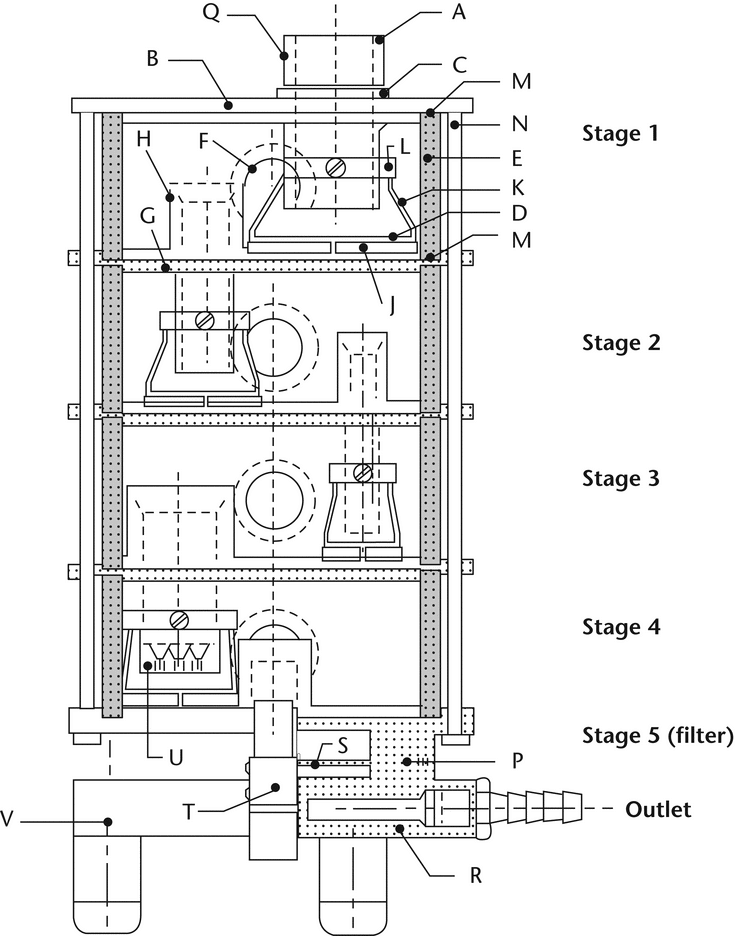
Fig. 8. Apparatus 4: Schematic of multistage liquid impinger. (See Table 4 for component specifications.)
Because the size distributions produced by different impactors are often a function of impactor design and the airflow rate through them, there is a need to standardize the instruments that are used to test inhalers (i.e., Apparatus 1 or 6 for metered-dose inhalers) or to provide guidelines on system suitability where different apparatuses may be used (i.e., Apparatus 2, 3, 4, or 5 for dry powder inhalers).
Because of the varied nature of the formulations and devices being tested, the cascade impaction system and technique selected for testing an inhaler should fulfill a number of criteria.
Stage Mensuration—
Manufacturers of cascade impaction devices provide a definitive calibration for the separation characteristics of each impaction stage in terms of the relationship between the stage collection efficiency and the aerodynamic diameter of particles and droplets passing through it as an aerosol. Calibration is a property of the jet dimensions, the spatial arrangement of the jet and its collection surface, and the airflow rate passing through it. Because jets can corrode and wear over time, the critical dimensions of each stage, which define that impaction stage's calibration, must be measured on a regular basis. This process, known as stage mensuration, replaces the need for repetitive calibration (using standard aerosols) and ensures that only devices that conform to specifications are used for testing inhaler output. The process involves the measurement and adjustment of the critical dimensions of the instrument.
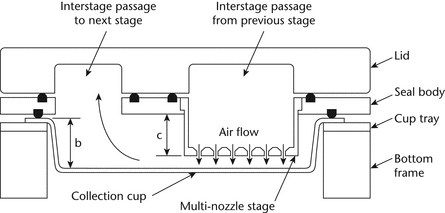
Interstage Drug Loss (wall losses)—
Where method variations are possible and there is no apparatus specified in the monograph, the selected technique should ensure that not more than 5% of the inhaler's total delivered drug mass (into the impactor) is subject to loss between the impaction device's sample collection surfaces. In the event that interstage drug losses are known to be greater than 5%, either the procedure should be performed in such a way that wall losses are included along with the associated collection plate, or an alternative apparatus should be used. As an example, the following procedures described for Apparatus 1 and 3 have been written to include wall losses along with the associated collection plate. Provided, however, that such losses are known to be less than or equal to 5% of the total delivered drug mass into the impactor and that there are no instructions to the contrary in an individual monograph, the technique may be simplified by only assaying drug on the collection plates.
Re-Entrainment—
Where method variations are possible, the selected technique should seek to minimize particle re-entrainment (from an upper to a lower impaction stage) on stages that contribute to size fractions defined in the individual monograph, especially where this may affect the amounts of drug collected. Minimizing the number of sampled doses, the use of coated particle collection surfaces, and proving that multiple-dose techniques produce statistically similar results to those from smaller numbers of doses, are all methods that can be used for this purpose. In the event that re-entrainment cannot be avoided, the number of doses collected, the time interval between doses, and the total duration of airflow through the cascade impaction device should be standardized. Under these circumstances, the presentation of impaction data should not presume the validity of the impactor's calibration (i.e., aerodynamic diameter ranges should not be assigned to drug masses collected on specific stages).
By using appropriate assay methods and a suitable mensurated impaction device, aerodynamic particle size distributions can be determined for drugs leaving the mouthpieces of metered-dose or dry powder inhalers. If temperature or humidity limits for use of the inhaler are stated on the label, it may be necessary to control the temperature and humidity of the air surrounding and passing through the device to conform to those limits. Ambient conditions are presumed, unless otherwise specified in individual monographs.
Mass Balance—
In addition to the size distribution, good analytical practice dictates that a mass-balance be performed in order to confirm that the amount of the drug discharged from the inhaler, which is captured and measured in the induction port-cascade impactor apparatus, is within an acceptable range around the expected value. The total mass of drug collected in all of the components (material balance) divided by the total number of minimum recommended doses discharged is not less than 75% and not more than 125% of the average minimum recommended dose determined during testing for Delivered-Dose Uniformity. This is not a test of the inhaler but serves to ensure that the test results are valid.
Use one of the multistage impaction devices shown below, or an equivalent, to determine aerodynamic particle size distributions of drugs leaving the mouthpieces of metered-dose or dry powder inhalers. Apparatus 1 and 6 [Figures 4 and 9 (without preseparator), respectively] are intended for use with metered-dose inhalers at a single airflow rate. Apparatus 2, 3, 4, and 5 (Figures 6, 7, 8, and 9, respectively) are intended for use with dry powder inhalers at the appropriate airflow rate, Qout, determined earlier, provided that the value of Qout falls in the range 30–100 L per minute.
Note—If Qout is greater than 100 L per minute, testing should be performed with Qout set at 100 L per minute; if Qout is less than 30 L per minute, testing is performed with Qout at 30 L per minute.
Apparatus 1 for Metered-Dose Inhalers—
Use this apparatus, or an equivalent, at a flow rate of 28.3 L per minute (±5%), as specified by the manufacturer of the cascade impactor.
Design—
The design and assembly of this apparatus and the induction port to connect the device to an inhaler are shown in Figures 4, 4a, and 4b1.
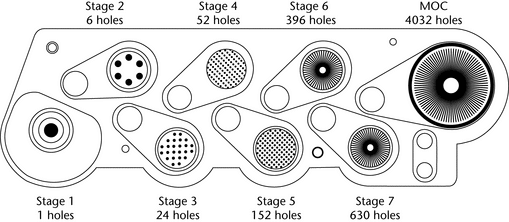
Critical engineering dimensions applied by manufacturers to the stages of Apparatus 1 are provided in Table 2. During use, some occlusion and blockage of jet nozzles may occur and therefore, “in use” mensuration tolerances need to be justified.
Table 2. Critical Dimensions for the Jet Nozzles of
Apparatus 1
Apparatus 1
| Stage # | Number of Jets | Nozzle Diameter (mm) |
|---|---|---|
| 0 | 96 | 2.55 ± 0.025 |
| 1 | 96 | 1.89 ± 0.025 |
| 2 | 400 | 0.914 ± 0.0127 |
| 3 | 400 | 0.711 ± 0.0127 |
| 4 | 400 | 0.533 ± 0.0127 |
| 5 | 400 | 0.343 ± 0.0127 |
| 6 | 400 | 0.254 ± 0.0127 |
| 7 | 201 | 0.254 ± 0.0127 |
Procedure—
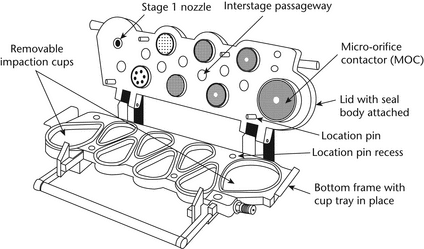
Set up the multistage cascade impactor as described in the manufacturer's literature with an after filter below the final stage to capture any fine particles that otherwise would escape from the device. To ensure efficient particle capture, coat the particle collection surface of each stage with glycerol, silicone oil, or other suitable liquid typically deposited from a volatile solvent, unless it has been demonstrated to be unnecessary. Attach the induction port and mouthpiece adapter to produce an airtight seal between the inhaler mouthpiece and the induction port as shown in Figure 4. Use a mouthpiece adapter that ensures that the tip of the inhaler mouthpiece is flush with the open end of the induction port. Ensure that the various stages of the cascade impactor are connected with airtight seals to prevent leaks. Turn on the vacuum pump to draw air through the cascade impactor, and calibrate the airflow through the system with an appropriate flowmeter attached to the open end of the induction port. Adjust the flow-control valve on the vacuum pump to achieve steady flow through the system at the required rate, and ensure that the airflow through the system is within ±5% of the flow rate specified by the manufacturer. Unless otherwise prescribed in the patient instructions, shake the inhaler for 5 seconds and discharge one delivery to waste. With the vacuum pump running, insert the mouthpiece into the mouthpiece adapter and immediately fire the minimum recommended dose into the cascade impactor. Keep the valve depressed for a duration sufficient to ensure that the dose has been completely discharged. If additional sprays are required for the sample, wait for 5 seconds before removing the inhaler from the mouthpiece adapter, shake the inhaler, reinsert it into the mouthpiece adapter, and immediately fire the next minimum recommended dose. Repeat until the required number of doses have been discharged. The number of minimum recommended doses discharged must be sufficient to ensure an accurate and precise determination of Aerodynamic Size Distribution. [Note—The number of minimum recommended doses is typically not greater than 10. ] After the last dose has been discharged, remove the inhaler from the mouthpiece adapter. Rinse the mouthpiece adapter and induction port with a suitable solvent, and dilute quantitatively to an appropriate volume. Disassemble the cascade impactor, place each stage and its associated collection plate or filter in a separate container, and rinse the drug from each of them. [Note—If it has been determined that wall losses in the impactor are less than or equal to 5%, then the collection plates only may be used. ]
Dilute each quantitatively to an appropriate volume. Using the method of analysis specified in the individual monograph, determine the mass of drug collected in each of the components. To analyze the data, proceed as directed under Data Analysis.
Apparatus 2 for Dry Powder Inhalers—
Design—
The design and assembly of Apparatus 2, and the induction port to connect the device to an inhaler, are shown in Figure 6.2 [Note—The induction port is shown in detail in Figure 4a. ] The impactor has five impaction stages and an after filter. At a volumetric airflow rate of 60 L per minute (the nominal flow rate, Qn), the cutoff aerodynamic diameters D50,Qn of Stages 1 to 5 are 10, 5, 2.5, 1.25, and 0.625 µm, respectively. The after filter effectively retains aerosolized drug in the particle size range up to 0.625 µm. Set up the multistage cascade impactor with the control system as specified in Figure 5. To ensure efficient particle capture, coat the particle collection surface of each stage with glycerol, silicone oil, or other suitable liquid typically deposited from a volatile solvent, unless it has been demonstrated to be unnecessary. Assemble the impactor as described in the manufacturer's literature with an after filter below the final stage to capture any fine particles that otherwise would escape from the device. Attach the induction port and mouthpiece adapter to produce an airtight seal between the inhaler mouthpiece and the induction port. Use a mouthpiece adapter that ensures that the tip of the inhaler mouthpiece is flush with the open end of the induction port. Ensure that the various stages of the cascade impactor are connected with airtight seals to prevent leaks.
Turn on the vacuum pump, open the solenoid valve, and calibrate the airflow through the system as follows. Connect a flowmeter to the induction port. Use a flowmeter calibrated for the volumetric flow leaving the meter to directly determine Qout, or, if such a meter is unobtainable, calculate the volumetric flow leaving the meter (Qout) using the ideal gas law. For example, for a meter calibrated for the entering volumetric flow (Qin), use the formula:
Qout = QinP0/(P0 – DP)
where P0 is the atmospheric pressure and DP is the pressure drop over the meter. Adjust the flow-control valve to achieve a steady flow through the system at the required rate, Qout, so that Qout is within ±5% of the value determined during testing for Delivered-Dose Uniformity. Ensure that critical flow occurs in the flow-control valve, at the airflow rate to be used during testing, by using the following procedure. With the inhaler in place, and the intended flow running, measure the absolute pressure on both sides of the flow-control valve (P2 and P3 in Figure 5). A ratio of P3/P2 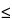 0.5 indicates critical flow. Switch to a more powerful pump, and remeasure the test flow rate if P3/P2 > 0.5. Adjust the timer controlling the operation of the two-way solenoid valve so that it opens this valve for a duration of T seconds as determined during testing for Delivered-Dose Uniformity. Prime or load the dry powder inhaler with powder for inhalation according to the labeled instructions. With the vacuum pump running and the two-way solenoid valve closed, insert the inhaler mouthpiece, held horizontally, into the induction port mouthpiece adapter. Discharge the powder into the apparatus by opening the two-way solenoid valve for a duration of T seconds. After the two-way solenoid valve has closed, remove the inhaler from the mouthpiece adapter. If additional doses are required for the sample, reload the inhaler according to the labeled instructions, reinsert the mouthpiece into the mouthpiece adapter, and repeat the operation until the required number of doses have been discharged. After discharge of the last dose, switch off the vacuum pump.
0.5 indicates critical flow. Switch to a more powerful pump, and remeasure the test flow rate if P3/P2 > 0.5. Adjust the timer controlling the operation of the two-way solenoid valve so that it opens this valve for a duration of T seconds as determined during testing for Delivered-Dose Uniformity. Prime or load the dry powder inhaler with powder for inhalation according to the labeled instructions. With the vacuum pump running and the two-way solenoid valve closed, insert the inhaler mouthpiece, held horizontally, into the induction port mouthpiece adapter. Discharge the powder into the apparatus by opening the two-way solenoid valve for a duration of T seconds. After the two-way solenoid valve has closed, remove the inhaler from the mouthpiece adapter. If additional doses are required for the sample, reload the inhaler according to the labeled instructions, reinsert the mouthpiece into the mouthpiece adapter, and repeat the operation until the required number of doses have been discharged. After discharge of the last dose, switch off the vacuum pump.
Rinse the mouthpiece adapter and induction port with a suitable solvent, and quantitatively dilute to an appropriate volume. Disassemble the cascade impactor, and place the after filter in a separate container. Rinse the drug from each of the stages and the filter, and quantitatively dilute each to an appropriate volume. Using the method of analysis specified in the individual monograph, determine the mass of drug collected in each of the components. Determine the cutoff diameters of each of the individual stages of the impactor, at the value of Q = Qout employed in the test by the formula:
D50,Q = D50,Qn(Qn/Q)½, (Eq. 1)
(Eq. 1)
where D50,Q is the cutoff diameter at the flow rate, Q, employed in the test, and the subscript, n, refers to the nominal values determined when Qn equals 60 L per minute. Thus, when Q equals 40 L per minute, the cutoff diameter of Stage 2 is given by the formula:
D50,40LPM = 5 µm × [60/40]1/2 = 6.1 µm.
General Procedure—
Perform the test using Apparatus 2 at the airflow rate, Qout determined earlier, during testing for Delivered-Dose Uniformity, provided Qout is less than or equal to 100 L per minute. [Note—If Qout is greater than 100 L per minute, use an airflow rate of 100 L per minute. ] Connect the apparatus to a flow control system that is based upon critical (sonic) flow as specified in Figure 5 (see also Table 3).
Table 3. Component Specifications for Figure 5
Component Specifications for Figure 5
| Code | Item | Description | Dimensions |
|---|---|---|---|
| A | Connector | (e.g., short metal coupling with low diameter branch to P3) | |
| B | Vacuum tubing | (e.g., silicon tubing with an outside diameter of 14 mm and an internal diameter of 8 mm) | A length of suitable tubing |
| C | Two-way solenoid valvea | See Fig. 5 | 2-way, 2-port solenoid valve having an ID |
| D | Vacuum pumpb | See Fig. 5 | Pump must be capable of drawing the required flow rate through the assembled apparatus with the dry powder inhaler in the mouthpiece adapter. Connect the pump to the solenoid valve using short and wide ( |
| E | Timerc | See Fig. 5 | The timer switches current directly to the solenoid valve for the required duration. |
| P2, P3 | Pressure measurements | Determine under steady-state flow conditions with an absolute pressure transducer. | |
| F | Flow control valved | See Fig. 5 | Adjustable regulating valve with maximum Cv |
|
a
An example being ASCO product number 8030G13 (Automatic Switch Company, 60 Hanover Road, Florham Park, NJ 07932) or equivalent. See also Footnote h in Table 1.
b
Gast product type 1023, 1423, or 2565 (Gast Manufacturing Inc., PO Box 97, Benton Harbor, MI 49022) or equivalent.
c
An example being Eaton Product number 45610-400 (Eaton Corporation, Automotive Products Division, 901 South 12th Street, Watertown, WI 53094) or equivalent.
d
Parker Hannifin type 8FV12LNSS, or equivalent (Parker Hannifin plc, Riverside Road, Barnstable, Devon EX31 1NP, UK). See also Footnote h in Table 1.
|
Table 4. Component Units of Multistage Liquid Impinger (see Fig. 8)
| Code1 | Item | Description | Dimensions2 |
|---|---|---|---|
| A,H | Jet tube | Metal tube screwed onto partition wall sealed by gasket (C), polished inner surface | see Figure 8a |
| B,G | Partition wall | Circular metal plate, diameter | 120 |
| Thickness | see Figure 8a | ||
| C | Gasket | e.g., PTFE | to fit jet tube |
| D | Impaction plate | Porosity O sintered-glass disk, | |
| Diameter | see Figure 8a | ||
| E | Glass cylinder | Plane polished cut glass tube | |
| Height, including gaskets | 46 | ||
| Outer diameter | 100 | ||
| Wall thickness | 3.5 | ||
| Sampling port (F) diameter | 18 | ||
| Stopper in sampling port | ISO 24/25 | ||
| J | Metal frame | l-profiled circular frame with slit | |
| Inner diameter | to fit impaction plate | ||
| Height | 4 | ||
| Thickness of horizontal section | 0.5 | ||
| Thickness of vertical section | 2 | ||
| K | Wire | Steel wire interconnecting metal frame and sleeve (two for each frame) | |
| Diameter | 1 | ||
| L | Sleeve | Metal sleeve secured on jet tube by screw | |
| Inner diameter | to fit jet tube | ||
| Height | 6 | ||
| Thickness | 5 | ||
| M | Gasket | e.g., silicone | to fit glass cylinder |
| N | Bolt | Metal bolt with nut (six pairs), length | 205 |
| Diameter | 4 | ||
| P | O-ring | Rubber O-ring, diameter × thickness | 66.34 × 2.62 |
| Q | O-ring | Rubber O-ring, diameter × thickness | 29.1 × 1.6 |
| R | Filter holder | Metal housing with stand and outlet | see Figure 8b |
| S | Filter support | Perforated sheet metal, diameter | 65 |
| Hole diameter | 3 | ||
| Distance between holes (center-points) | 4 | ||
| T | Snap-locks | ||
| U | Multi-jet tube | Jet tube (H) ending in multijet arrangement | see inserts Figure 8a |
| V | Outlet | Outlet and nozzle for connection to vacuum | Internal diameter |
|
1
See Fig. 8.
2
Measurements in mm unless otherwise stated.
|
|||
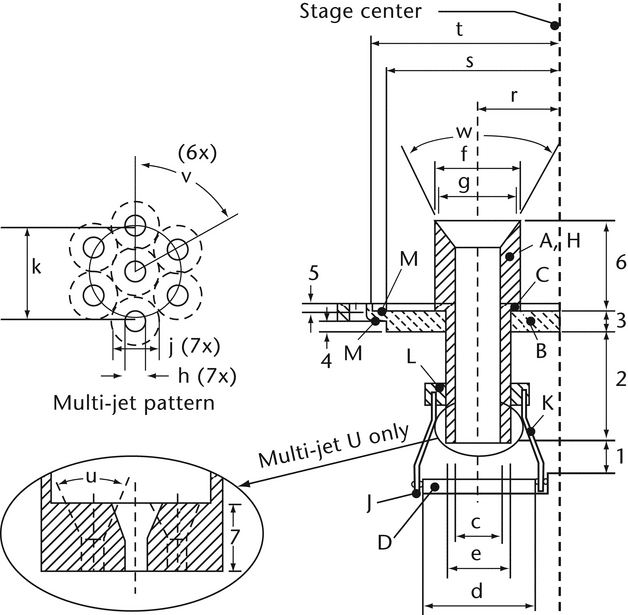
Fig. 8a. Apparatus 4: Details of jet tube and impaction plate. Inserts show end of multi-jet tube U leading to Stage 4. (See Table 5 for dimension specifications.)
Fig. 8b. Apparatus 4: Expanded view of Stage 5. (See Table 4 for component specifications.)
| Type | Code2 | Stage 1 | Stage 2 | Stage 3 | Stage 4 | Filter (Stage 5) |
|---|---|---|---|---|---|---|
| Distance | 1 | 9.5 ( |
5.5 ( |
4.0 ( |
6.0 ( |
n.a. |
| Distance | 2 | 26 | 31 | 33 | 30.5 | 0 |
| Distance | 3 | 8 | 5 | 5 | 5 | 5 |
| Distance | 4 | 3 | 3 | 3 | 3 | n.a. |
| Distance | 5 | 0 | 3 | 3 | 3 | 3 |
| Distance | 63 | 20 | 25 | 25 | 25 | 25 |
| Distance | 7 | n.a. | n.a. | n.a. | 8.5 | n.a. |
| Diameter | c | 25 | 14 | 8.0(±0.1) | 21 | 14 |
| Diameter | d | 50 | 30 | 20 | 30 | n.a. |
| Diameter | e | 27.9 | 16.5 | 10.5 | 23.9 | n.a. |
| Diameter | f | 31.75 ( |
22 | 14 | 31 | 22 |
| Diameter | g | 25.4 | 21 | 13 | 30 | 21 |
| Diameter | h | n.a. | n.a. | n.a. | 2.70 (±.05) | n.a. |
| Diameter | j | n.a. | n.a. | n.a. | 6.3 | n.a. |
| Diameter | k | n.a. | n.a. | n.a. | 12.6 | n.a. |
| Radius4 | r | 16 | 22 | 27 | 28.5 | 0 |
| Radius4 | s | 46 | 46 | 46 | 46 | n.a. |
| Radius4 | t | n.a. | 50 | 50 | 50 | 50 |
| Angle | w | 10 |
53 |
53 |
53 |
53 |
| Angle | u | n.a. | n.a. | n.a. | 45 |
n.a. |
| Angle | v | n.a. | n.a. | n.a. | 60 |
n.a. |
|
1
Measurements in mm with tolerances according to ISO 2768-m, unless otherwise stated.
2
See Fig. 8a.
3
Including gasket.
4
Relative centerline of stage compartment.
n.a.: not applicable. |
Under steady flow conditions, at the appropriate volumetric airflow rate through the entire apparatus, ensure that critical (sonic) flow occurs in the flow control valve by determining the individual values for absolute pressure, P2 and P3, so that their ratio P3/P2 is less than or equal to 0.5. Coat the particle collection surface of each of the stages of the cascade impactor to ensure that particles that have impacted on a given stage are not re-entrained in the flowing airstream, unless this has been shown to be unnecessary. Analyze the data as directed under Data Analysis.
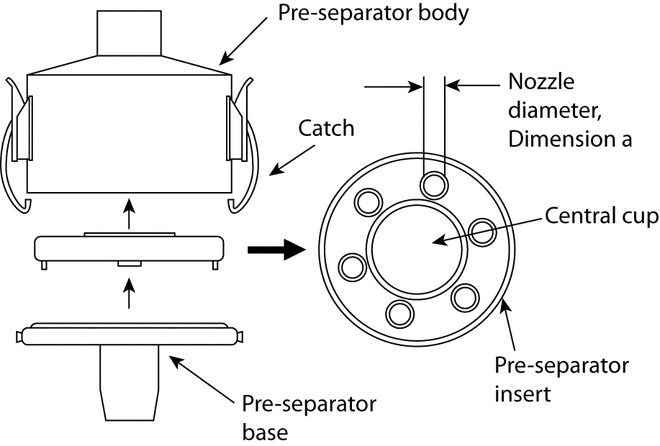
Apparatus 3 for Dry Powder Inhalers—
Design—
Apparatus 3 is identical to Apparatus 1 (Figure 4), except that the manufacturer's preseparator is added atop Stage 0 to collect large masses of noninhalable powder prior to their entry into the impactor, and the outlet nipple, used to connect to vacuum tubing B (Figure 5), is replaced with one having an internal diameter 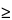 8 mm. To connect the preseparator of the impactor to the induction port (Figure 4a), a specially designed top for the preseparator must be used. This is shown in Figure 7.3 The impactor, therefore, has eight stages, a preseparator (to collect large particulates), and an after filter. At a volumetric airflow rate of 28.3 L per minute (the nominal flow rate, Qn), the cutoff aerodynamic diameters D50,Qn of Stages 0 to 7 are 9.0, 5.8, 4.7, 3.3, 2.1, 1.1, 0.7, and 0.4 µm, respectively. The after filter effectively retains aerosolized drug in the particle size range up to 0.4 µm. Connect the cascade impactor into the control system specified in Figure 5. Omit Stage 6 and Stage 7 from the impactor if the test flow rate, Qout, used during testing for Delivered-Dose Uniformity was greater than or equal to 60 L per minute. To ensure efficient particle capture, coat the particle collection surface of each stage with glycerol, silicone oil, or other suitable liquid typically deposited from a volatile solvent, unless it has been demonstrated to be unnecessary. Assemble the impactor as described in the manufacturer's literature with an after filter below the final stage to capture any fine particles that otherwise would escape from the device. Place an appropriate volume (up to 10 mL) of an appropriate solvent into the preseparator, or coat the particle collection surfaces of the preseparator to prevent re-entrainment of impacted particles. [Caution—Some solvents form flammable vapor-air mixtures that may be ignited during passage through a vacuum pump. Take appropriate precautions (alternative solvents, use of vapor traps, minimal pump operating times, etc.) to ensure operator safety during testing.
] Attach a molded mouthpiece adapter to the end of the induction port to produce an airtight seal between the inhaler mouthpiece and the induction port. Use a mouthpiece adapter that ensures that the tip of the inhaler mouthpiece is flush with the open end of the induction port. Ensure that the various stages of the cascade impactor are connected with airtight seals to prevent leaks.
8 mm. To connect the preseparator of the impactor to the induction port (Figure 4a), a specially designed top for the preseparator must be used. This is shown in Figure 7.3 The impactor, therefore, has eight stages, a preseparator (to collect large particulates), and an after filter. At a volumetric airflow rate of 28.3 L per minute (the nominal flow rate, Qn), the cutoff aerodynamic diameters D50,Qn of Stages 0 to 7 are 9.0, 5.8, 4.7, 3.3, 2.1, 1.1, 0.7, and 0.4 µm, respectively. The after filter effectively retains aerosolized drug in the particle size range up to 0.4 µm. Connect the cascade impactor into the control system specified in Figure 5. Omit Stage 6 and Stage 7 from the impactor if the test flow rate, Qout, used during testing for Delivered-Dose Uniformity was greater than or equal to 60 L per minute. To ensure efficient particle capture, coat the particle collection surface of each stage with glycerol, silicone oil, or other suitable liquid typically deposited from a volatile solvent, unless it has been demonstrated to be unnecessary. Assemble the impactor as described in the manufacturer's literature with an after filter below the final stage to capture any fine particles that otherwise would escape from the device. Place an appropriate volume (up to 10 mL) of an appropriate solvent into the preseparator, or coat the particle collection surfaces of the preseparator to prevent re-entrainment of impacted particles. [Caution—Some solvents form flammable vapor-air mixtures that may be ignited during passage through a vacuum pump. Take appropriate precautions (alternative solvents, use of vapor traps, minimal pump operating times, etc.) to ensure operator safety during testing.
] Attach a molded mouthpiece adapter to the end of the induction port to produce an airtight seal between the inhaler mouthpiece and the induction port. Use a mouthpiece adapter that ensures that the tip of the inhaler mouthpiece is flush with the open end of the induction port. Ensure that the various stages of the cascade impactor are connected with airtight seals to prevent leaks.
Turn on the vacuum pump, open the two-way solenoid valve, and calibrate the airflow through the system as follows. Prime or load the dry powder inhaler with powder for inhalation according to the labeled instructions. With the vacuum pump running and the two-way solenoid valve closed, insert the inhaler mouthpiece, held horizontally, into the induction port mouthpiece adapter. Once the inhaler is positioned, discharge the powder into the apparatus by activating the timer and opening the two-way solenoid valve for the required duration, T ± 5%, as determined during testing for Delivered-Dose Uniformity. After the two-way solenoid valve has closed, remove the inhaler from the mouthpiece adapter. If additional doses are required for the sample, reload the inhaler according to the labeled instructions, reinsert the mouthpiece into the mouthpiece adapter, and repeat the operation until the required number of doses have been discharged. After discharge of the last dose, remove the inhaler from the mouthpiece adapter, and switch off the vacuum pump.
Carefully disassemble the apparatus. Using a suitable solvent, rinse the drug from the mouthpiece adapter, induction port, and preseparator, and quantitatively dilute to an appropriate volume. Rinse the drug from each stage, and the impaction plate immediately below, into appropriately sized flasks. Quantitatively dilute each flask to an appropriate volume. Using the method of analysis specified in the individual monograph, determine the mass of drug collected in each of the samples. The aerodynamic cutoff diameters of the individual stages of this device, in the airflow range between 30 and 100 L per minute, are currently not well established. Do not use the formula in Equation 1 to calculate cutoff diameters.
Procedure—
Proceed as directed in the General Procedure under Apparatus 2, except to use Apparatus 3.
Apparatus 4 for Dry Powder Inhalers—
Note—Apparatus 4, the multistage liquid impinger, has a small number of stages and is used extensively outside the USA. It is provided here for the benefit of users in countries other than the USA.
Design—
The design and assembly of Apparatus 4 are shown in Figs. 8, 8a, and 8b.4 The induction port, used to connect the device to an inhaler, is shown in Fig. 4a. The device is a multi-stage liquid impinger consisting of impaction Stages 1, 2, 3, and 4 and an integral after filter (Stage 5). The collection stages of the liquid impinger (see Fig. 8 and Table 4) are kept moist, unlike those of traditional impactors, such as Apparatus 1, 2, 3, 5, and 6; wetting may produce an effect similar to coating the stages of Apparatus 2, 3, 5, and 6 at certain flow rates, although this should be confirmed by demonstrating control over re-entrainment as described earlier. An impaction stage comprises an upper horizontal metal partition wall (B) through which a metal inlet jet tube (A) with its impaction plate (D) is protruding; a glass cylinder (E) with sampling port (F), forming the vertical wall of the stage; and a lower horizontal metal partition wall (G) through which a jet tube (H) connects to the lower stage. The tube into Stage 4 (U) ends in a multi-jet arrangement. The impaction plate (D) is secured in a metal frame (J), which is fastened by two wires (K) to a sleeve (L) secured on the jet tube (C). For more detail of the jet tube and impaction plate, see Fig. 8a. The horizontal plane of the collection plate is perpendicular to the axis of the jet tube and centrally aligned. The upper surface of the impaction plate is slightly raised above the edge of the metal frame. A recess around the perimeter of the horizontal partition wall guides the position of the glass cylinder. The glass cylinders are sealed against the horizontal partition walls with gaskets (M) and clamped together by six bolts (N). The sampling ports are sealed by stoppers. The bottom side of the lower partition wall of Stage 4 has a concentric protrusion fitted with a rubber O-ring (P) that seals against the edge of a filter placed in the filter holder. The filter holder (R) is a basin with a concentric recess in which a perforated filter support (S) is flush-fitted. The filter holder is designed for 76-mm diameter filters. The whole impaction stage assembly is clamped onto the filter holder by two snap locks (T). The impinger is equipped with an induction port (Fig. 4a) that fits onto the Stage 1 inlet jet tube. A rubber O-ring on the jet tube provides an airtight connection to the induction port. An elastomeric mouthpiece adapter to fit the inhaler being tested provides an airtight seal between the inhaler and the induction port.
At a volumetric airflow rate of 60 L per minute (the nominal flow rate, Qn), the cutoff aerodynamic diameters D50,Qn of Stages 1 to 4 are 13.0, 6.8, 3.1, and 1.7 µm, respectively. The after filter effectively retains aerosolized drug in the particle size range up to 1.7 µm. Ensure that Apparatus 4 is clean and free of drug solution from any previous tests. Place a 76-mm diameter filter in the filter stage, and assemble the apparatus. Use a low pressure filter capable of quantitatively collecting the passing drug aerosol, which also allows a quantitative recovery of the collected drug. Set up Apparatus 4 using the control system as specified in Figure 5. Attach the induction port (Figure 4a) and mouthpiece adapter to produce an airtight seal between the inhaler mouthpiece and the induction port. Use a mouthpiece adapter that ensures that the tip of the inhaler mouthpiece is flush with the open end of the induction port. Ensure that the various stages of the apparatus are connected with airtight seals to prevent leaks. Turn on the vacuum pump, open the two-way solenoid valve, and calibrate the airflow through the system as follows. Connect a flowmeter, calibrated for the volumetric flow rate leaving the meter, to the induction port. Adjust the flow-control valve to achieve a steady flow through the system at the required rate, Qout, so that Qout is within ±5% of the value determined during testing for Delivered-Dose Uniformity. Ensure that critical flow occurs in the flow-control valve, at the value of Qout to be used during testing, using the following procedure. With the inhaler in place, and the intended flow running, measure the absolute pressure on both sides of the flow-control valve (P2 and P3 in Figure 5). A ratio of P3/P2 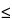 0.5 indicates critical flow. Switch to a more powerful pump, and remeasure the test flow rate if P3/P2 > 0.5. Adjust the timer controlling the operation of the two-way solenoid valve so that it opens that valve for the same duration, T, as used during testing for Delivered-Dose Uniformity. Dispense 20 mL of a solvent, capable of dissolving the drug, into each of the four upper stages of Apparatus 4, and replace the stoppers. [Caution—Some solvents form flammable vapor-air mixtures that may be ignited during passage through a vacuum pump. Take appropriate precautions (alternative solvents, use of vapor traps, minimal pump operating times, etc.) to ensure operator safety during testing.
] Tilt the apparatus to wet the stoppers, thereby neutralizing their electrostatic charge. Adjust the timer controlling the operation of the two-way solenoid valve so that it opens the valve for the same duration, T, as used during testing for Delivered-Dose Uniformity. Prime or load the dry powder inhaler with powder for inhalation according to the labeled instructions. With the vacuum pump running and the two-way solenoid valve closed, insert the inhaler mouthpiece, held horizontally, into the induction port mouthpiece adapter. Discharge the powder into the apparatus by activating the timer and opening the two-way solenoid valve for the required duration, T ± 5%. After the two-way solenoid valve has closed, remove the inhaler from the mouthpiece adapter. If additional doses are required for the sample, reload the inhaler according to the labeled instructions, reinsert the mouthpiece into the mouthpiece adapter, and repeat the operation until the required number of doses have been discharged. After discharge of the last dose, switch off the vacuum pump.
0.5 indicates critical flow. Switch to a more powerful pump, and remeasure the test flow rate if P3/P2 > 0.5. Adjust the timer controlling the operation of the two-way solenoid valve so that it opens that valve for the same duration, T, as used during testing for Delivered-Dose Uniformity. Dispense 20 mL of a solvent, capable of dissolving the drug, into each of the four upper stages of Apparatus 4, and replace the stoppers. [Caution—Some solvents form flammable vapor-air mixtures that may be ignited during passage through a vacuum pump. Take appropriate precautions (alternative solvents, use of vapor traps, minimal pump operating times, etc.) to ensure operator safety during testing.
] Tilt the apparatus to wet the stoppers, thereby neutralizing their electrostatic charge. Adjust the timer controlling the operation of the two-way solenoid valve so that it opens the valve for the same duration, T, as used during testing for Delivered-Dose Uniformity. Prime or load the dry powder inhaler with powder for inhalation according to the labeled instructions. With the vacuum pump running and the two-way solenoid valve closed, insert the inhaler mouthpiece, held horizontally, into the induction port mouthpiece adapter. Discharge the powder into the apparatus by activating the timer and opening the two-way solenoid valve for the required duration, T ± 5%. After the two-way solenoid valve has closed, remove the inhaler from the mouthpiece adapter. If additional doses are required for the sample, reload the inhaler according to the labeled instructions, reinsert the mouthpiece into the mouthpiece adapter, and repeat the operation until the required number of doses have been discharged. After discharge of the last dose, switch off the vacuum pump.
Dismantle the filter stage of Apparatus 4. Carefully remove the filter, and extract the drug with solvent. Rinse the mouthpiece adapter and induction port with a suitable solvent, and quantitatively dilute to an appropriate volume. Rinse the inside of the inlet jet tube to Stage 1 (Figure 8), allowing the solvent to flow into the stage. Rinse the drug from the inner walls and the collection plate of each of the four upper stages of the apparatus, into the solution in the respective stage, by tilting and rotating the apparatus, while ensuring that no liquid transfer occurs between the stages. Using the method of analysis specified in the individual monograph, determine the mass of drug collected in each of the six volumes of solvent. Ensure that the method corrects for possible evaporation of the solvent during the test. This may involve the use of an internal standard (of known original concentration in the solvent and assayed at the same time as the drug) or the quantitative transfer of the liquid contents from each of the stages, followed by dilution to a known volume. Determine the cutoff diameters of each of the individual stages of the impactor, at the value of Q = Qout employed in the test by the formula:
D50,Q = D50,Qn (Qn/Q)1/2
where D50,Q is the cutoff diameter at the flow rate, Q, employed in the test, and the subscript, n, refers to the nominal values determined when Qn equals 60 L of air per minute. Thus, when Q equals 40 L of air per minute, the cutoff diameter of Stage 2 is given by the formula:
D50,40LPM = 6.8 µm × (60/40)1/2 = 8.3 µm.
Procedure—
Proceed as directed in the General Procedure under Apparatus 2, except to use Apparatus 4.
Apparatus 5 for Dry Powder Inhalers—
Design—
The design and assembly of Apparatus 55 are shown in Figures 9, 9a, 9b, 9c, and 9d. The induction port, used to connect the device to an inhaler, is shown in Figure 4a. The device is a cascade impactor with seven stages and a micro-orifice collector (MOC). Over the design flow-rate range of 30 to 100 L per minute, the 50% efficiency cut-off diameters of the stages (D50 values) range between 0.24 µm to 11.7 µm, evenly spaced on a logarithmic scale. In the design flow-rate range, there are always at least five stages with D50 values between 0.5 µm and 6.5 µm. The collection efficiency curves for each stage are sharp and minimize overlap between stages. Material may be aluminum, stainless steel, or other suitable material.
The impactor layout has removable impaction cups with all the cups in one plane (Figures 9–9c). There are three main sections to the impactor: the bottom frame that holds the impaction cups, the seal body that holds the jets, and the lid that contains the interstage passageways (shown in Figures 9–9b). Multiple nozzles are used at all but the first stage (Figure 9c). The flow passes through the impactor in a saw-tooth pattern.
Stage mensuration is performed periodically together with confirmation of other dimensions critical to the effective operation of the impactor. Critical dimensions are provided below in Table 6.
Table 6. Critical Dimensions for Apparatus 5 and 6
| Description | Dimension (mm) |
|---|---|
| Preseparator (dimension a—see Figure 9d) | 12.80 ± 0.05 |
| Stage 11 Nozzle diameter | 14.30 ± 0.05 |
| Stage 21 Nozzle diameter | 4.88 ± 0.04 |
| Stage 31 Nozzle diameter | 2.185 ± 0.02 |
| Stage 41 Nozzle diameter | 1.207 ± 0.01 |
| Stage 51 Nozzle diameter | 0.608 ± 0.01 |
| Stage 61 Nozzle diameter | 0.323 ± 0.01 |
| Stage 71 Nozzle diameter | 0.206 ± 0.01 |
| MOC 1 | approximately 0.070 |
| Cup Depth (Dimension b—see Figure 9b) | 14.625 ± 0.10 |
| Collection cup surface roughness | 0.5 to 2 µm |
| Stage 1 Nozzle to seal body distance2—dimension c | 0 ± 1.18 |
| Stage 2 Nozzle to seal body distance2—dimension c | 5.236 ± 0.736 |
| Stage 3 Nozzle to seal body distance2—dimension c | 8.445 ± 0.410 |
| Stage 4 Nozzle to seal body distance2—dimension c | 11.379 ± 0.237 |
| Stage 5 Nozzle to seal body distance2—dimension c | 13.176 ± 0.341 |
| Stage 6 Nozzle to seal body distance2—dimension c | 13.999 ± 0.071 |
| Stage 7 Nozzle to seal body distance2—dimension c | 14.000 ± 0.071 |
| MOC Nozzle to seal body distance2—dimension c | 14.429 – 14.571 |
|
1
See Figure 9c.
2
See Figure 9b.
|
|
In routine operation, the seal body and lid are held together as a single assembly. The impaction cups are accessible when this assembly is opened at the end of an inhaler test. The cups are held in a support tray, so that all cups can be removed from the impactor simultaneously by lifting out the tray.
An induction port with internal dimensions identical to those defined in Figure 4a is connected to the impactor inlet. When necessary, with dry powder inhalers, a preseparator can be added to avoid overloading the first stage. This preseparator connects between the induction port and the impactor. A suitable mouthpiece adapter is used to provide an airtight seal between the inhaler and the induction port.
At a volumetric airflow rate of 60 L per minute (the assigned reference flow rate for cutoff-diameter calculations, Qn), the cutoff-aerodynamic diameters D50,Qn of Stages 1 to 7 are 8.06, 4.46, 2.82, 1.66, 0.94, 0.55 and 0.34 µm, respectively. The apparatus contains a terminal micro-orifice collector (MOC) that for most formulations may eliminate the need for a final filter as determined by method validation. The MOC is an impactor nozzle plate and collection cup. The nozzle plate contains, nominally, 4032 jets, each approximately 70 µm in diameter. Most particles not captured on Stage 7 of the impactor will be captured on the cup surface below the MOC. (For impactors operated at 60 L per minute, the MOC is capable of collecting 80% of 0.14-µm particles). For formulations with a significant fraction of particles not captured by the MOC, there is an optional filter holder that can replace the MOC or be placed downstream of the MOC containing a suitable after-filter (glass fiber is often suitable).
Procedure—
Assemble the apparatus with the preseparator (Figure 9d), unless experiments have shown that its omission does not result in increased interstage drug losses (>5%) or particle re-entrainment, in which case the preseparator may be omitted.
Place cups into the apertures in the cup tray. To ensure efficient particle capture, coat the particle collection surface of each stage with glycerol, silicone oil, or other suitable liquid typically deposited from a volatile solvent, unless it has been demonstrated to be unnecessary. Insert the cup tray into the bottom frame, and lower into place. Close the impactor lid with the seal body attached, and operate the handle to lock the impactor together so that the system is airtight.
The preseparator may be assembled as follows: assemble the preseparator insert into the preseparator base; fit the preseparator base to the impactor inlet; add 15 mL of the solvent used for sample recovery to the central cup of the preseparator insert; place the preseparator body on top of this assembly; and close the two catches. [Caution—Some solvents form flammable vapor-air mixtures that may be ignited during passage through a vacuum pump. Take appropriate precautions (e.g., alternative solvents, use of vapor traps, minimal pump operating times, etc.) to ensure operator safety during testing.
]
Connect an induction port with internal dimensions as defined in Figure 4a either to the impactor inlet or to the preseparator inlet atop the cascade impactor (Figure 9d). Place a suitable mouthpiece adapter in position at the end of the induction port so that the mouthpiece end of the inhaler, when inserted, lines up along the horizontal axis of the induction port. The front face of the inhaler mouthpiece is flush with the front face of the induction port, producing an airtight seal. When attached to the mouthpiece adapter, the inhaler should be positioned in the same orientation as intended for use. Connect the apparatus to a flow system according to the scheme specified in Figure 5.
Unless otherwise prescribed, conduct the test at the flow rate used in the test for Delivered-Dose Uniformity drawing 4 L of air from the mouthpiece of the inhaler and through the apparatus. Connect a flowmeter to the induction port. Use a flowmeter calibrated for the volumetric flow leaving the meter, or calculate the volumetric flow leaving the meter (Qout) using the ideal gas law. For a meter calibrated for the entering volumetric flow (Qin), use the formula:
Qout = QinP0/(P0 – DP)
where P0 is the atmospheric pressure and DP is the pressure drop over the meter. Adjust the flow control valve to achieve steady flow through the system at the required rate, Qout (±5%). Ensure that critical flow occurs in the flow-control valve by the procedure described for Apparatus 2. Adjust the timer controlling the operation of the two-way solenoid valve so that it opens the valve for the same duration, T, as used during testing for Delivered-Dose Uniformity.
Prime or load the dry powder inhaler with powder for inhalation according to the labeled instructions. With the vacuum pump running and the two-way solenoid valve closed, insert the inhaler mouthpiece, held horizontally, into the induction port mouthpiece adapter. Discharge the powder into the apparatus by activating the timer and opening the two-way solenoid valve for the required duration, T(±5%). After the two-way solenoid valve has closed, remove the inhaler from the mouthpiece adapter. If additional doses are required for the sample, reload the inhaler according to the labeled instructions, reinsert the mouthpiece into the mouthpiece adapter, and repeat the operation until the required number of doses have been discharged. After discharge of the last dose, switch off the vacuum pump.
Dismantle the apparatus, and recover drug for analysis as follows: remove the induction port and mouthpiece adapter from the preseparator and extract the drug into an aliquot of solvent; if used, remove the preseparator from the impactor, without spilling the solvent into the impactor; and recover the active ingredient from all inner surfaces.
Open the impactor by releasing the handle and lifting the lid. Remove the cup tray, with the collection cups, and recover the active ingredient from each cup into an aliquot of solvent. Using the method of analysis specified in the individual monograph, determine the mass of drug contained in each of the aliquots of solvent.
Determine the cutoff diameters of each of the individual stages of the impactor, at the value of Q = Qout employed in the test by the formula:
D50,Q = D50,Qn (Qn/Q)X, (Eq. 2)
(Eq. 2)
where D50,Q is the cutoff diameter at the flow rate, Qemployed in the test, and the subscript, n, refers to the nominal or reference value for Qn = 60 L of air per minute (see Table 7). The values for the exponent, x, are listed in Table 7. Thus, when Q = 40 L of air per minute, the cutoff diameter of Stage 2 is given by the formula:
D50,40LPM = 4.46 µm × (60/40)0.52 = 5.51 µm.
Analyze the data as directed under Data Analysis.
Table 7. Cutoff Aerodynamic Diameter for Stages of
Apparatus 5 and 6
Apparatus 5 and 6
| Use Eq. 2 to calculate D50,Q for flow rates, Q, in the range 30 to 100 L per minute with Qn = 60 L per minute. | ||
|---|---|---|
| Stage | D50,Qn | x |
| 1 | 8.06 | 0.54 |
| 2 | 4.46 | 0.52 |
| 3 | 2.82 | 0.50 |
| 4 | 1.66 | 0.47 |
| 5 | 0.94 | 0.53 |
| 6 | 0.55 | 0.60 |
| 7 | 0.34 | 0.67 |
Apparatus 6 for Metered-Dose Inhalers—
Design—
Apparatus 6 is identical to Apparatus 5 (Figures 9-9d), except that the preseparator is not to be used. Use this apparatus at a flow rate of 30 L per minute (±5%), unless otherwise prescribed in the individual monograph.
Procedure—
Assemble the apparatus without the preseparator. Place cups into the apertures in the cup tray. To ensure efficient particle capture, coat the particle collection surface of each stage with glycerol, silicone oil, or other suitable liquid typically deposited from a volatile solvent, unless it has been demonstrated to be unnecessary. Insert the cup tray into the bottom frame, and lower into place. Close the impactor lid with seal body attached, and operate the handle to lock the impactor together so that the system is airtight. Connect an induction port with internal dimensions as defined in Figure 4a to the impactor inlet. Use a mouthpiece adapter that ensures that the tip of the inhaler mouthpiece is flush with the open end of the induction port. Turn on the vacuum pump to draw air through the cascade impactor, and calibrate the airflow through the system with an appropriate flowmeter attached to the open end of the induction port. Adjust the flow-control valve on the vacuum pump to achieve steady flow through the system at the required rate, and ensure that the airflow through the system is within ±5% of this flow rate. Unless otherwise prescribed in the patient instructions, shake the inhaler for 5 seconds, and discharge one delivery to waste. With the vacuum pump running, insert the mouthpiece into the mouthpiece adapter, and immediately fire the minimum recommended dose into the cascade impactor. Keep the valve depressed for a duration sufficient to ensure that the dose has been completely discharged. If additional sprays are required for the sample, shake the inhaler, reinsert it into the mouthpiece adapter, and immediately fire the next minimum recommended dose.
Repeat until the required number of doses have been discharged. The number of minimum recommended doses discharged must be sufficient to ensure an accurate and precise determination of Aerodynamic Size Distribution. [Note—The number of minimum recommended doses is typically not greater than 10. ] After the last dose has been discharged, remove the inhaler from the mouthpiece adapter. Rinse the mouthpiece adapter and induction port with a suitable solvent, and dilute quantitatively to an appropriate volume.
Dismantle the apparatus, and recover the drug for analysis as follows: remove the induction port and mouthpiece adapter from the apparatus, and recover the deposited drug into an aliquot of solvent; open the impactor by releasing the handle and lifting the lid; remove the cup tray, with the collection cups; and extract the active ingredient in each cup into an aliquot of solvent. Using the method of analysis specified in the individual monograph, determine the quantity of active ingredient contained in each of the aliquots of solvent.
Determine the cutoff diameters of each of the individual stages of the impactor, at the value of Q employed in the test by using Eq. 2 with values obtained from Table 7. Thus, when Q = 30 L of air per minute, the cutoff diameter of Stage 2 is given by the formula:
D50,30LPM = 4.46 µm × (60/30)0.52 = 6.40 µm.
To analyze the data, proceed as directed under Data Analysis.
Data Analysis
This section describes the data analysis required to define the Aerodynamic Size Distribution of the drug output from the test inhaler, after the use of Apparatus 1, 2, 3, 4, 5, or 6. Enter the data collected from Apparatus 1, 2, 3, 4, 5, or 6 in the table of mass summaries as shown in Table 8. Perform only those calculations specified in the individual monograph.
Table 8. Table of Mass Summaries for Analyses of Metered-Dose Inhalers and Dry Powder Inhalers
| Mass | Apparatus 1 | Apparatus 2 | Apparatus 3a | Apparatus 4b | Apparatus 5d | Apparatus 6d | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mouthpiece adapter | Ai | — | AiAi | — | Ai | — | Ai | — | Ai | — | Ai | — |
| Preseparator | — | — | — | — | AP | — | — | — | AP | — | — | — |
| Stage 0 of impactor | A0 | B0 | — | — | A0 | B0 | — | — | — | — | — | — |
| Stage 1 of impactor/impinger | A1 | B1 | A1 | — | A1 | B1 | A1 | — | A1 | B1 | A1 | B1 |
| Stage 2 of impactor/impinger | A2 | B2 | A2 | B2 | A2 | B2 | A2 | B2 | A2 | B2 | A2 | B2 |
| Stage 3 of impactor/impinger | A3 | B3 | A3 | B3 | A3 | B3 | A3 | B3 | A3 | B3 | A3 | B3 |
| Stage 4 of impactor/impinger | A4 | B4 | A4 | B4 | A4 | B4 | A4 | B4 | A4 | B4 | A4 | B4 |
| Stage 5 of impactor/impinger | A5 | B5 | A5 | B5 | A5 | B5 | — | — | A5 | B5 | A5 | B5 |
| Stage 6 of impactor/impinger | A6 | B6 | — | — | A6 | B6 | — | — | A6 | B6 | A6 | B6 |
| Stage 7 of impactor/impinger | A7 | B7 | — | — | A7 | B7 | — | — | A7 | B7 | A7 | B7 |
| Filter | AF | BF | AF | BF | AF | BF | AF | BF | AF | BF | AF | BF |
| Sums of Masses | SAc | SBc | SAc | SBc | SAc | SBc | SAc | SBc | SAc | SBc | SAc | SBc |
|
a
Stages 6 and 7 are omitted from Apparatus 3 at airflow rates >60 L per minute.
b
Stage 5 of Apparatus 4 is the filter stage (see Figure 8).
c
SA is the total drug mass recovered from the apparatus; SB is the mass of drug recovered from the impactor (Apparatus 1, 3, 5 and 6) or from the impactor stages beneath the uppermost stage (Apparatus 2 and 4).
d
For Apparatus 5 and 6, values for the drug masses AF and BF refer to collections from the MOC, and/or the after-filter if used.
|
||||||||||||
calculations
Fine Particle Dose and Fine Particle Fraction—
Calculate the total mass, SA, of drug delivered from the mouthpiece of the inhaler into the apparatus. Then calculate the total mass, R, of drug found on the stages of the apparatus and the filter that captured the drug in the fine particle size range appropriate for the particular drug being tested. The Fine Particle Dose is calculated by the formula:
R/n
where R is as stated above, and n is the number of doses discharged during the test. The Fine Particle Fraction that would be delivered from the inhaler is then calculated by the formula:
R/SA.
Cumulative Percentage (Cum%) of Drug Mass Less Than Stated Aerodynamic Diameter—
Construct Table 9 by dividing the mass of drug on the filter stage by SB (see Table 8). Multiply the quotient by 100, and enter this number as a percentage opposite the effective cutoff diameter of the stage immediately above it in the impactor or impinger stack. For Apparatus 2 or 4, use Equation 1 to calculate the stage cutoff diameters, D50,Q, at the airflow rate, Q, employed during the test. For Apparatus 5 and 6, use Equation 2 with Table 7. For Apparatus 1, use the cutoff diameters quoted by the manufacturer. For Apparatus 3, present the data as cumulative percentages of mass on and below the stated stage, and avoid assigning values to stage cutoff diameters.
Repeat the calculation for each of the stages in the impactor or impinger stack, in reverse numerical order (largest to smallest stage number). For each stage, calculate the cumulative percentage of mass less than the stated aerodynamic diameter by adding the percentage of the mass on that stage to the total percentage from the stages below and entering the value opposite the effective cutoff diameter of the stage above it in the stack. Thus, the percentage of drug on the filter can be seen to have aerodynamic diameters less than the cutoff diameter of the stage above the filter, and the percentage on the filter plus the percentage on the stage above have diameters less than the cutoff diameter of the stage above that, and so on. Repeat the calculation for each of the remaining stages in reverse numerical order (see Table 9).
Table 9. Cumulative Percentage (Cum%) of Mass Less than the Stated Aerodynamic Diameter
| Apparatus 1 | Apparatus 2 | Apparatus 3a | Apparatus 4b | Apparatus 5 | Apparatus 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mass | Cum%c | D50d | Cum%c | D50,Qd | Cum%c | D50,Qe | Cum%c | D50,Qd | Cum%c | D50,Qd | Cum%c | D50,Qd |
| Filter | 0.4 | 0.625 | 0.4 | 1.7 | 0.34 | 0.34 | ||||||
| Stage 7 | b | 0.7 | — | — | b | 0.7 | — | — | b | 0.55 | b | 0.55 |
| Stage 6 | c | 1.1 | — | — | c | 1.1 | — | — | c | 0.94 | c | 0.94 |
| Stage 5 | d | 2.1 | b | 1.25 | d | 2.1 | — | — | d | 1.66 | d | 1.66 |
| Stage 4 | e | 3.3 | c | 2.5 | e | 3.3 | b | 3.1 | e | 2.82 | e | 2.82 |
| Stage 3 | f | 4.7 | d | 5.0 | f | 4.7 | c | 6.8 | f | 4.46 | f | 4.46 |
| Stage 2 | g | 5.8 | 100 | 10.0 | g | 5.8 | 100 | 13.0 | g | 8.06 | g | 8.06 |
| Stage 1 | h | 9.0 | — | — | h | 9.0 | — | — | — | —— | — | — |
| Stage 0 | 100 | — | — | — | 100 | — | — | — | 100 | — | 100 | — |
|
a
Stages 6 and 7 are omitted from Apparatus 3 at flow rates >60 L per minute; thus, values for b and c should be omitted for Apparatus 3, where necessary.
b
The filter stage in Apparatus 4 is Stage 5 (see Figure 8).
c
[(mass on stage / SB)×100] % + (total% of SB from stages below).
d
The 50% cutoff diameter of the stage immediately above that indicated (e.g., for Stage 4, enter the cutoff diameter for Stage 3; for Apparatus 2 or 4, calculate as D50,Q from Eq. 1; for Apparatus 5 or 6, calculate as D50,Q from Eq. 2 using Table 7). Values entered in the Table are correct for Apparatus 1, 2, 4, 5, and 6 only when used at 28.3, 60.0, 60.0, 60.0, and 60.0 L per minute, respectively.
e
The D50 values are only valid at a flow rate of 28.3 L per minute.
|
||||||||||||
If necessary, and where appropriate, plot the percentage of mass less than the stated aerodynamic diameters, versus the aerodynamic diameter, D50,Q, on log probability paper. Calculate the GSD by the equation:
Use these data and/or plot to determine values for MMAD and GSD etc., as appropriate and when necessary (see Figure 10).
1
A suitable cascade impactor is available as Model Mk II from Thermo-Electron, 27 Forge Parkway, Franklin, MA 02038. The impactor is used without the preseparator. The inhaler is connected to the impactor via the induction port, atop the entrance cone shown in Figure 4. If an equivalent impactor is employed, the induction port in Figure 4a should be used, although the entrance cone (Fig. 4b) should be replaced with one to fit the impactor in question. Note that the internal surfaces of the induction port (Fig. 4a) are designed to fit flush with their counterparts in the entrance cone (Fig. 4b). This design avoids aerosol capture at the junction of the two pipes.
2
The cascade impactor is available as the Model 160 Marple-Miller Impactor from MSP Corporation, Minneapolis, MN. The inhaler should be connected to the impactor via the induction port, shown in Figure 4a.
3
The cascade impactor is available as the Andersen 1ACFM Non-Viable Cascade Impactor (Mark II) from Thermo-Electron, 27 Forge Parkway, Franklin, MA 02038. The impactor is used with the preseparator.
4
The five-stage impinger is available from Copley Instruments, plc, Nottingham, UK. The inhaler should be connected to the impactor via the induction port, shown in Fig. 4 and Fig. 4a.
5
The cascade impactor is available as the Next Generation Pharmaceutical Impactor from MSP Corporation, Minneapolis, MN.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Kahkashan Zaidi, Ph.D.
Senior Scientific Liaison 1-301-816-8269 |
(GCDF2010) General Chapters - Dosage Forms |
USP35–NF30 Page 232
Pharmacopeial Forum: Volume No. 37(4)