INTRODUCTION
This general chapter provides a comprehensive overview of considerations for the development of cellular and tissue-based products. A collection of terms commonly used in this field is provided under Glossary and Definition of Terms. Cell and tissue-based therapies are medical products that contain human or animal cells that will be administered to humans to repair, replace, regenerate, or augment a recipient’s cells, tissue, or organs that are diseased, dysfunctional, or injured. The source cells or tissue can be harvested for use without manipulation or may be propagated, expanded, pharmacologically treated, or otherwise altered in biological characteristics ex vivo before administration. The diversity of clinical indications and types of cell and tissue-based products are shown in Table 1.
Table 1. Examples of Cell-Based Therapy Products
| Indication | Product |
|---|---|
| Hematopoietic stem cell transplantation following ablative therapy |
Hematopoietic stem and progenitor cells that have been harvested, propagated, selected, and/or treated for removal of contaminating cells by means of devices and/or reagents |
| Cancer | T cells, NK cells, dendritic cells, or macrophages exposed to cancer-specific peptides to elicit an anticancer response; autologous or allogeneic cancer cells, genetically or biochemically modified and irradiated to elicit an anticancer response |
| Diabetes | Encapsulated |
| Myocardial infarction | Autologous or allogeneic stem/progenitor cells; skeletal myocytes; cardiac-derived stem cells |
| Graft-versus-host disease | Allogeneic mesenchymal stem cells |
| Wound healing | Autologous keratinocytes or allogeneic dermal fibroblasts on a biocompatible scaffold |
| Focal defects in knee cartilage | Autologous or allogeneic chondrocytes with or without a biocompatible scaffold |
| Bone repair | Mesenchymal stem cells in a biocompatible scaffold |
| Neurodegenerative diseases | Neuronal progenitor cells derived from embryonic, fetal, or adult source tissues; cells genetically modified to secrete neurotrophic factors, with or without encapsulation |
| Infectious disease | Activated T-cells |
| Autoimmune disease | Regulatory T-cells (Treg) |
| Spinal cord injury | Neuronal progenitor cells |
| Organ repair or regeneration | Autologous or allogeneic cells on biocompatible biomaterials (gels) or 3-dimensional scaffold structure |
Cell therapy products can be modified by treatment with integrating or nonintegrating genetic materials (DNA, RNA, siRNA, etc.) so that the pattern of gene expression is changed. Typically, cells are taken from the patient and are modified outside of the body before they are returned to the patient. Regulatory bodies consider the ex vivo gene-modified cellular product to be a gene therapy product. A great deal of information in this general chapter is relevant to processing, characterization, manufacturing, and administration of genetically modified cells. However detailed information about the use of various gene transfer systems, patient monitoring considerations, genetic analysis, and other issues pertinent to gene therapy products are addressed in Gene Therapy Products  1047
1047 .
.
This general chapter describes issues related to the manufacturing, sourcing of components, and characterization of cellular or tissue-based products to ensure their safety and efficacy. A list of relevant regulatory and guidance documents is presented in the Appendix. Manufacturers of cellular or tissue-based products should consider and apply the controls and procedures outlined in this chapter to ensure the products’ safe use in humans. New methodologies are continually being developed and validated and will be included in the United States Pharmacopeia (USP) as they become available. USP monographs for specific tissue and tissue-based products outline test specifications that should be met throughout a product’s time in the market place. The term cellular product refers to living human or animal cells or tissues that have been manipulated or are used in ways that result in their regulation as somatic cellular therapies, as defined by the US Food and Drug Administration (FDA). A tissue-based product refers to human tissues subject to regulation under good tissue practices (GTPs). Combination products refer to cells combined with medical devices, such as a natural or synthetic scaffold.
Considerations for Incorporating Quality System Concepts Early in Cellular and Tissue-Based Product Development
Current and future regulatory requirements will continue to challenge developers of cellular and tissue-based products to incorporate robust quality attributes early in the design phase to ensure a focus on patient safety by means of a high degree of process understanding. Modern quality systems that harmonize current Good Manufacturing Practices (cGMPs) with other non-US pharmaceutical regulatory systems [such as the International Conference on Harmonization (ICH) and the International Organization for Standardization (ISO)] and the FDA medical device quality system are being recognized as the new global standards. These new standards include product development concepts such as Quality by Design (QbD) and Process Analytical Technology (PAT). Moreover, these quality systems integrate approaches to continual improvement and risk management that promote adoption of the latest scientific advances and innovative manufacturing technologies.
Employing the principles of Quality Risk Management (QRM) early in product development may identify areas of risk that can be mitigated before they are incorporated into the manufacturing process and affect the safety and efficacy of the product. Developers of cell and tissue-based products should employ risk management and assessment techniques as a key component of their quality systems. Risk management is defined as a systematic process for the identification, assessment, and control of risks to the quality of the cell or tissue-based product across the product lifecycle. Using QRM techniques can help achieve safe and efficacious products by assessing patient risks, determining design space boundaries, or ranking quality attributes. QRM can also establish and maintain a state of control by using risk management to drive process control. Finally, QRM can be used to facilitate continual improvement by prioritizing opportunities for improvement. The level of effort, formality, and documentation of the risk management process should be commensurate with the level of risk, should be based on scientific knowledge, and ultimately should be linked to patient protection.
The elements of risk management have become an accepted paradigm and can be readily accessed in FDA and international regulatory guidance documents, especially ICH Q9. A number of tools have been developed to facilitate this assessment. These tools provide a quantifiable means of prioritizing risk so that higher-risk elements of a process can be identified and corrected.
Depending on the objective of the risk management program, risk analysis can be more or less formalized. Preliminary, less formal risk analysis comes into play when a risk assessment needs to be expedited, as in the resolution of a manufacturing nonconformance. A more formalized risk assessment system is necessary for process or product development. This is especially important when limited resources must be prioritized. Formalized systems are predicated on well-established tools that can quantify risk in every phase or step of manufacturing. These systems can also be used in evaluating raw material choices, validation prioritization, facility alterations, equipment changes, and utility deliberations.
Formal risk analysis tools include process mapping, preliminary hazard analysis, Hazard Analysis of Critical Control Points (HACCP), Hazard Operability Analysis (HAZOP), Fault Tree Analysis (FTA), Failure Mode Effects Analysis (FMEA), and Failure Mode Effects and Criticality Analysis (FMECA).
For cell and tissue-based products, FMEA has been commonly used to identify, quantify, and prioritize risk. FMEA can assign a numerical rating in one of three categories:
- Severity, which is the consequence of a failure;
- Occurrence, which is the likelihood of the failure happening based on past experience or nonconformance; and
- Detection, based on the ability to detect the failure.
Each category is assigned a numerical rating (typically 1 to 5 or 1 to 10) consistent with the severity of the excursion from the operating parameter range, the probability of an excursion, and the likelihood of detecting an excursion before it has an effect on the product. Lower numbers refer to an unlikely probability of detection whereas higher numbers refer to the likelihood of a failure or hazardous effect. The product of the severity, occurrence, and detection values is a Risk Priority Number (RPN). In the risk-evaluation process RPNs are prioritized, and the most immediate remediation can be directed to areas of highest risk.
COMPONENTS USED IN CELL AND TISSUE-BASED PRODUCT MANUFACTURING
Introduction
Manufacturers of cellular or tissue-based products must ensure that all components used in manufacturing are appropriately qualified. Examples of components used in the production of cellular or tissue-based therapies include the source cells and tissues; natural or synthetic biomaterials; ancillary materials required during manufacturing but not intended to be present in the final therapeutic product; and excipients used in the formulation of cellular or tissue-based therapies.
Qualification is the process of acquiring and evaluating data to establish the source, identity, purity, biological safety, and overall suitability of a specific component to ensure quality. The diversity of cellular and tissue-therapy products and of the materials used to produce them makes it difficult to recommend specific tests or protocols for a qualification program. Therefore, rational and scientifically sound programs must be developed for each component.
Material qualification activities will change as products move from clinical trials to licensure and commercialization. A well-designed qualification program becomes more comprehensive as product development progresses. In the early stages of product development, safety concerns should be the primary focus of a material qualification plan. In the later stages, material qualification activities should be completely developed and should comply with cGMP.
Qualification of Source Cells and Tissues
Various human- and animal-derived cells and tissues serve as source material for cell and tissue-based products. Three sources of donor cells for cell-therapy products include:
- The patient’s own cells (autologous cell products)
- Cells from another human being (allogeneic cell products)
- Cells derived from animals (xenogeneic cell products)
The source of cells used for a particular cell or tissue-based therapy largely depends on compatibility, purity, and availability. Use of autologous cells has the advantage of minimal concerns regarding immune rejection. However, an autologous source is not always available and appropriate if the cell type is dysfunctional, malignant, not readily obtainable, or contaminated.
The alternative is a compatible allogeneic cell source that may be more readily available. Of primary concern with the use of allogeneic cell sources is immune incompatibility, which could lead to rejection of the administered cell or tissue-based therapy. In immunocompromised recipients, the donor cells may react to the patient’s cells, leading to graft-versus-host disease, which can be life threatening. Despite the potential complications of using allogeneic donor cells or tissues, in the absence of other alternatives the risk-to-benefit ratio is often favorable. A number of approaches successfully circumvent immune barriers for the use of allogeneic sources. Immunosuppressive drugs developed for solid organ transplantation and advances in inducing immune tolerance are increasingly applied to cell transplantation. Certain allogeneic cells elicit minimal immune reactions, even in HLA-mismatched recipients. Examples include mesenchymal stem cells, certain dermal and epidermal cells, and fibroblasts.
Despite advances in the derivation of new types of therapeutic cells, particularly stem cells (adult, fetal, embryonic and induced pluripotent cells), the ability to generate certain types of cells or tissues remains elusive. As a result, ongoing efforts use xenogeneic cells and tissues to treat a number of human diseases or conditions. Use of xenogeneic cells must address concerns about both immune rejection and transmission of animal viruses to humans (see Animal Sources of Cells and Tissues, below).
Some general principles in the sourcing of tissues include: (1) systems must allow the material to be traced back to the donor, while adhering to privacy legislation; (2) steps must be taken to prevent the transmission of infectious diseases from the donor to the recipient; and (3) aseptic procurement and processing must ensure the safety of the final product because terminal sterilization of products containing living cells and tissues is not possible. FDA has promulgated a specific set of regulations, referred to as GTPs, that specifically address the need to procure and process tissues in a manner that avoids transmission of a communicable disease. GTPs and/or GMPs must be followed for cell or tissue-based therapy products, depending on cell source and place in the product life cycle.
donor eligibility
FDA has enacted a comprehensive set of regulations governing human tissues and human cells that are intended for implantation, transplantation, infusion, or transfer into a human recipient. These materials are referred to as human cells, tissues, or cellular or tissue-based products (HCT/Ps). Paramount for procurement of HCT/Ps for medical use is adherence to donor eligibility requirements. These dictate that a donor’s relevant medical records must be reviewed to evaluate risk factors and clinical evidence of communicable disease agents. This includes obtaining a health history and performing a physical examination on a donor to screen for communicable diseases. In addition, donors must also undergo appropriate laboratory testing using FDA-cleared or -approved test kits for specific relevant communicable disease agents and diseases (RCDADs). Required disease testing will expand as new RCDADs are identified and FDA-approved or -cleared test kits become available. Two sources for information about communicable disease testing are FDA’s Guidance on Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products and AABB’s Circular of Information (http://www.aabb.org/Content/About_Blood/Circulars_of_Information/aabb_coi.htm). Donor eligibility determination is not required for autologous HCT/Ps.
human cells, tissues, or cellular or tissue-based products
HCT/Ps may be sourced from normal healthy donors, cadaveric donors, or patients with diseases such as cancer. The suitability of tissue sourced from patients with cancer and other diseases should be assessed before collection to ensure adequate safety and function of the final cell therapy product. Additionally, the regulations in 45 CFR Part 46 apply to all federally supported human subject research. These regulations require that an Institutional Review Board review and approve the use of any tissue taken from a human donor. The regulations also include special considerations for research on prisoners, children, pregnant women, or gestational tissue. In all cases appropriate written consent must be obtained from the donor or the donor’s next of kin describing the tissue that is being procured and its intended use.
The risk of disease transmission to the manufacturing operator should be minimized by appropriate training for handling potentially infectious materials and by the use of protective equipment and clothing. Tissues should be obtained under environmental conditions and controls that provide a high degree of assurance for aseptic recovery.
Hematopoietic progenitor cells (HPCs) are one of the most extensively used cell sources for human transplantation. These cells can be collected from the bone marrow, peripheral blood, or umbilical cord blood. The source of cells depends on the patient, the disease, and the clinical protocol. Regardless of the cell source, methods for processing the cells are similar. HPCs can be sourced from healthy donors or patients with hematological disorders. In addition to FDA’s HCT/Ps regulations, applicable guidelines and standards for the collection and processing of these materials have been published by the American Association of Blood Banks (AABB), the Foundation for the Accreditation of Hematopoietic Cell Therapy, and the National Marrow Donor Program (NMDP).
For cell or tissue sources obtained from surgical specimens or cadaveric donors, standard hospital operating room practices are applicable. The air quality in a typical limited-access operating room is adequate for such procedures. Procurement personnel must be appropriately trained in all aspects of tissue recovery, such as surgical scrubbing, gowning, operating room behavior, anatomy, surgical site preparation, and aseptic technique. Special care is required when tissue or organ procurement requires extensive manipulation of the bowel, which may result in the inadvertent puncture of the bowel. Tissue that contains microbial flora (e.g., skin) at the time of procurement can be adequately disinfected with antimicrobial or bactericidal agents and extensive scrubbing.
animal sources of cells and tissue
Ideally, cellular therapy products would consist of human cells manufactured with minimal exposure to animal-based materials. However, at present important unmet medical needs may potentially be addressed by cellular therapy products from animal cells or tissues. One example is pancreatic islets intended to treat diabetes. Human sources of pancreatic islets are available only from pancreas donated at the time of death. The quality of donor organ islets is variable, and the available supply is inadequate to meet potential demand. One approach is procurement of pancreatic islets from appropriately qualified animal sources for subsequent use in humans (xenotransplantation).
Developers who intend to use animal cells or tissues in a cellular therapy product must adequately address public health concerns and must develop approaches to mitigate the potential risk of introduction and propagation of zoonotic infectious agents into the general human population. The PHS Guideline on Infectious Disease Issues in Xenotransplantation (January 2001) describes potential risks. The FDA guidance Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans (April 2003) reflects updated approaches and expectations to minimize risks of xenogeneic cellular products.
The use of animal tissue in the manufacture of cell therapy products requires that the tissue be sourced in a controlled and documented manner from designated pathogen-free animals bred and raised in captivity in countries or geographic regions that have appropriate disease prevention and control systems. In addition, the care and use of animals should be approved by a certified institutional animal care and use committee. Donor animals must have documented lineage, be obtained from closed herds or colonies, and be under health maintenance and monitoring programs. The animal housing facility should be USDA certified (large vertebrate animals) or Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) certified (small vertebrate animals) and should meet the recommendations stated in the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996), which can be obtained from AAALAC (www.aaalac.org). Such facilities should be staffed with veterinarians and other trained personnel who ensure animal health and disease prevention. The facility’s procedures should be documented, and records should be kept. Health maintenance and monitoring programs should be based on standard veterinary care for the species, including physical examinations, monitoring, laboratory diagnostic tests, and vaccinations. A stepwise batch or all-in–all-out method of source animal movement through the facility can minimize the potential for transmission of infectious agents.
Feed components should be documented and should exclude recycled or rendered materials in order to reduce the risk of prion-associated diseases.
To provide a high degree of assurance of product safety, animal donors and tissues should be screened at several stages throughout the process to rule out the presence of microbial agents. These control tests should utilize assays that are sufficiently sensitive and specific to detect bacteria, mycoplasma, fungi, or viruses of interest. Donor animals should be screened for relevant diseases before tissue procurement. Post-tissue-retrieval necropsies, sentinel animal programs, and archival storage of donor organs, tissues, blood, and other specimens also ensure the safety of animal tissue for use in cellular therapeutic applications.
In general, similar aseptic procurement issues apply to animal and human tissues. The tissue should be obtained under environmental conditions and controls that provide a high degree of assurance of aseptic recovery. Specifically designed procurement facilities, usually closely associated with the animal holding facility, should be employed. Recommended design features and attributes of the animal tissue procurement facility should include the following: (1) staging of events such as shaving, sedation, and operating room preparation in separate rooms with appropriate environmental controls; (2) high-efficiency particulate air (HEPA) filtration; (3) adjacent but separate facilities for further tissue processing; and (4) dedicated areas for carcass removal. Issues relating to personnel training, bowel manipulation and puncture, and disinfection apply to the surgical procurement of both human and animal tissues (see Human Cells, Tissues, or Cellular or Tissue-Based Products, above). When researchers establish animal cell lines for use as feeder layer cells, cell banks should be created, tested, and characterized as described in the next section.
cell bank system
A cell bank is a collection of cells obtained from pooled cells or derived from a single cell clone or donor tissue that is stored in bags or vials under defined conditions that maintain genotypic and phenotypic stability. The cell bank system usually consists of a master cell bank (MCB) and a working cell bank (WCB), although alternative approaches are possible. The MCB is produced in accordance with cGMP and preferably is obtained from a qualified source (one that is free from adventitious agents) with known and documented history. Human cells and tissues should be obtained by means of a licensed tissue acquisition vendor with a donor qualification program in accordance to 21 CFR 1271. The WCB is produced or derived by expanding one or more vials of the MCB. The WCB, or MCB in early trials, becomes the source of cells for every batch produced for human use. Cell bank systems contribute greatly to production batch consistency because the starting cell material is always the same. However, it may not be possible or feasible to create a cell bank, so appropriately tested and qualified primary cells may be used in lieu of creation of cell banks. The MCB and WCB should be minimally tested for identity, sterility, purity, viability, and the presence of viruses and mycoplasma.
cell bank qualification
Cell bank safety testing and characterization are important steps toward obtaining a uniform final product with lot-to-lot consistency and freedom from adventitious agents. ICH Q5A, Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin, gives specific recommendations for testing cell banks for viral agents. While this guideline is not specifically intended to cover cell or tissue-based products, the same tests are generally applicable. Additional virus testing may be needed depending on the prevalence of viral diseases endemic in the donor population. Testing to qualify the MCB is performed once and can be done on an aliquot of the banked material or on cell cultures derived from the cell bank. Specifications for qualification of the MCB should be prospectively established. It is important to document the MCB history, the methods and reagents used to produce the bank, and the storage conditions. All the ancillary materials required for production of the banks, such as media, sera, cytokines, growth factors, and enzymes, should also be qualified, documented, and appropriately tested.
safety testing of mcb and wcb
characterization of mcb and wcb
Characterization of the MCB and WCB includes identity testing to establish species origin, e.g., isoenzyme analyses to confirm the human origin of the cells. However, cell bank characterization should encompass additional assessments such as the following:
- Growth kinetics and population doubling time
- Morphological assessment
- Percent confluence at passage
- Cell counts
- Viability (pre- and postcryopreservation)
- Phenotypic expression of desired and undesired cell types (pre- and postcryopreservation)
- Monitoring of unique biochemical markers (pre- and postcryopreservation)
- Assessments of functional activity (pre- and postcryopreservation)
- Gene and protein expression analysis (pre- and postcryopreservation)
- Expression of immune histocompatibility antigens (HLA/M HC)
- Molecular fingerprinting
- Chromosomal stability
Biocompatible Scaffold Materials
Most natural or synthetic scaffold materials are regulated as medical devices, although scaffolds derived from human tissues such as dermis are regulated as HCT/Ps. When possible, use scaffolds that have previously been approved for other clinical uses because these materials should have already undergone extensive safety and quality testing. For applications in cell or tissue-based products, the scaffold material should allow cells to attach, proliferate, and migrate, and high porosity is often desired to facilitate cell seeding within the material. The scaffold must provide adequate diffusion of nutrients for cell health and release of cell-excreted products. The material must have adequate mechanical strength and must be amenable to manipulation, chemical modification, and manufacture. The scaffold material should be biocompatible, relatively inert, and immunologically benign.
Scaffolds can generally be classified as hard or soft. Hard scaffolds are used in applications where a specific shape is required, such as forming a blood vessel or a bladder. Soft scaffolds are used in applications where the product needs to conform flexibly to an existing shape in the body.
Scaffold materials can be synthetic or natural polymers, biodegradable or permanent. Biodegradation allows the scaffold to be resorbed or removed from the body without manipulation. The scaffold degradation rate must coincide with the rate of formation or regeneration of the tissue. The natural scaffold structure must replace the degrading scaffold in such a way that it maintains the structural integrity of the tissue or organ being regenerated. For example, a newly formed blood vessel must withstand both the internal blood pressure as well as external mechanical forces.
The most commonly used synthetic biodegradable polymer is polyglycolic acid (PGA). Polylactic acid (PLA) is also widely used, sometimes in combination with PGA. These polymers degrade within the body, are readily removed before degradation, and have a long history of use in suture materials. Polycaprolactum (PCL), which exhibits a slower rate of degradation than PLA or PGA, is used in applications that require a long presence in the body.
Extracellular matrix (ECM) and its derivatives are natural materials used for scaffolds in the manufacture of cell–biomaterial combination products. Proteins such as collagen or fibrin and polysaccharides such as chitosan or glycosaminoglycans (GAGs) have also been used in growing cells to make combination products. Collagen is by far the most popular substrate for cells and has been molded into scaffolds for a variety of products, mainly in tissue-engineered skin applications. Cross-linking agents such as glutaraldehyde and water-soluble carbodiimides have been used to enhance the strength of natural scaffolds. Depending on the source of the material, natural scaffolds can be immunogenic.
When cells must proliferate after seeding, the scaffold and the supporting culture system must allow the exchange of nutrients and waste products. A thick, impermeable matrix will lead to regions of necrotic tissue. Many tissue devices are designed so that they can eventually be removed from the patient.
The safety and biocompatibility of the scaffold and product-contact materials must be established. A full battery of tests recommended by Biological Reactivity Tests, In Vitro  87
87 , Biological Reactivity Tests, In Vivo
, Biological Reactivity Tests, In Vivo  88
88 , ISO 10993, or FDA Blue Book G95-1 should be performed. Process residuals and degradation products from the preparation of the scaffold should be quantified and limits should be established. The stability and storage conditions of scaffold materials should be established.
, ISO 10993, or FDA Blue Book G95-1 should be performed. Process residuals and degradation products from the preparation of the scaffold should be quantified and limits should be established. The stability and storage conditions of scaffold materials should be established.
Qualification of Ancillary Materials
Ancillary products include a wide variety of raw materials and components used in manufacturing. They may include relatively simple materials or complex substances such as culture media, buffers, growth factors, cytokines, cultivation and processing components, monoclonal antibodies, and cell-separation devices.
Ancillary materials are not intended to be present in the final therapeutic product. Defined media formulations typically include components such as albumin and transferrin that are purified from animal or human sources. The purification, processing, and extensive testing of such components further minimize—but do not eliminate—the risk of viral or microbial contamination. Residual amounts of ancillary materials used in the manufacturing process, including recombinant proteins or other defined media components, may be potentially antigenic so their removal from the final product should be assessed, and appropriate limits should be established when necessary.
Known risks are associated with the use of ancillary materials in the production of cell-therapy products. The quality of ancillary materials used in the production of a cellular therapy product can affect the safety, potency, and purity of the product. Ideally, each ancillary material employed in the manufacture of a cellular or tissue-therapy product should be produced under conditions that are in compliance with cGMP. However, complex or unique substances essential for process control or production may not be available from suppliers that produce them in compliance with cGMP. In these situations, the cellular or tissue-therapy product manufacturer should develop a scientifically sound strategy for qualifying the raw material. A qualification program for ancillary materials used in cell and tissue therapy manufacturing should address each of the following areas: (1) identification and selection, (2) suitability for use in manufacturing, (3) characterization and acceptance criteria, (4) vendor qualification, and (5) quality assurance. Lot history files should be constructed for each ancillary material.
Conformance to established specifications should be compared to the data supplied on the Certificate of Analysis. Traceability is essential, and lot numbers for each ancillary material used should be noted in the productions records of the cell-based product. USP general information chapter Ancillary Materials for Cell, Gene, and Tissue-Engineered Products  1043
1043 should be consulted for specific information about implementing an appropriate qualification program for these materials. Other USP chapters provide considerations about the qualification of specific ancillary materials (e.g., Bovine Serum
should be consulted for specific information about implementing an appropriate qualification program for these materials. Other USP chapters provide considerations about the qualification of specific ancillary materials (e.g., Bovine Serum  1024
1024 , Fetal Bovine Serum—Quality Attributes and Functionality Tests
, Fetal Bovine Serum—Quality Attributes and Functionality Tests  90
90 , and Growth Factors and Cytokines Used in Cell Manufacturing
, and Growth Factors and Cytokines Used in Cell Manufacturing  92
92 ).
).
Qualification of Excipients
During the final steps in the manufacturing process, excipients or substances that increase the stability of the therapeutic cells may be included. Examples of excipients include culture media, USP saline or other electrolyte solutions approved for injection, exogenous proteins such as human serum albumin, or cryoprotectants such as dimethyl sulfoxide (DMSO). Excipients are not intended to exert a direct therapeutic effect upon the patient; rather they are intended to contribute to maintenance of the quality attributes of the final cellular product. Because excipients will be administered to the patient along with the cells, particular attention must be paid to their qualification. In general, excipients that are already FDA approved for human use should be used whenever possible. If nonapproved excipients are used, a complete safety assessment should be done. For novel excipients such as cryopreservation solutions, appropriately designed preclinical safety studies may be needed.
MANUFACTURING OF CELL OR TISSUE-BASED PRODUCTS
Introduction
The manufacturing of cell or tissue-based products requires a number of operations and manipulations by individuals who are well trained in aseptic processing techniques. The technical competence of the personnel is particularly crucial to product safety and efficacy. Autologous products present more challenges for cell and tissue processing because lot segregation, line clearance, and operational processes must be developed to decrease the chance of mix-up of patient-specific lots (see Facility Design and Operation Considerations, below).
Cell Isolation and Selection
The general principles for processing human or animal tissues following aseptic procurement are independent of the cell or tissue source. The manufacture of cell products may occur at a cell manufacturing facility located in close proximity to the clinical site or at a distant central cell-manufacturing facility. The source cellular or tissue material should be packaged in sterile, leak-proof containers and transported from the procurement area to the processing area under controlled conditions that maintain cell viability. The fluid medium in which the specimens are bathed during transportation should be optimized to maintain cell and tissue viability. This transport medium can be supplemented with antibiotics. The antibiotic levels in process buffers should be decreased and eventually eliminated during subsequent processing steps so that antibiotics are not present in the final cellular product. In the case of blood products or tissues containing substantial amounts of blood, the transport media or buffered electrolyte solution should contain an anticoagulant.
isolation
Solid organs or tissues are usually dissected to expose a desired region. This material may be used as is for transplantation, or it may be further processed. If multicellular organoids (for instance, islets of Langerhans) or single-cell suspensions are desired, the tissue may be subjected to mechanical or enzymatic disaggregation. Physical disaggregation may be accomplished by the use of instruments that homogenize the material by imparting high shear forces or breaking the tissue into smaller pieces. Alternatively, the material can be pressed or passed through screens of defined mesh sizes.
Enzymatic digestion of the extracellular connective tissue is another common method for dissociating solid tissue. Various enzymes are used to accomplish this, including collagenase, dispase, trypsin, elastase, hyaluronidase, papain, and chymotrypsin. Enzymes with nuclease activity, such as deoxyribonuclease, may be added to digest nucleic acids released from damaged cells, preventing excessive cell clumping. At the end of the incubation process, the cell suspension may be subjected to a mild pumping action to further break up multicellular clusters into those of desired size or composition. Enzymatic and physical disaggregation methods are often combined to achieve the desired result.
Because blood and bone marrow cells are inherently suspensions, mechanical manipulation is usually limited to plasma and aggregate removal, which is accomplished by centrifugation and filtration.
Cell and tissue isolation activities involving open manipulation steps should be carried out in an ISO 5 (class 100) biological safety cabinet. The environment surrounding the biological safety cabinet should be suitable to maintain aseptic processing operations. For minimally manipulated HCT/Ps in closed systems, these environments may be controlled but unclassified. However, for cell and tissue-based therapies that are manipulated and manufactured under cGMPs, the environment surrounding the biological safety cabinet should be controlled and classified, usually as an ISO 7 (class 10,000) clean room. Precautions should be taken to segregate patient-specific tissue and cell isolates.
selection
Cell suspensions often consist of a mixture of cell types that may require further processing in order to isolate a cell population of interest or to decrease the level of an undesirable cell type such as potentially contaminating tumor cells. Various cell isolation and separation techniques provide high yields of pure cell populations.
Cell populations can be selectively enriched by varying the force and duration of centrifugation. Separation can also be achieved by isopycnic centrifugation in which the cell suspension is centrifuged in a gradient medium that encompasses all of the densities of cells in the sample. Specifically designed continuous-flow elutriation centrifuges separate cell populations by subjecting a cell suspension to opposite centrifugal and fluid stream forces in a special chamber within the centrifuge rotor mechanism. Cell populations separate within the rotor on the basis of their various sizes and densities, and they are selectively eluted out of the rotor chamber by increasing the fluid stream force. Finally, other methods involve the addition of high-density agents such as hydroxyethyl starch to the cell suspension. Concentration and separation procedures such as these frequently result in cell loss because of clumping and aggregation.
Cell separation can also be achieved by applying techniques that take advantage of unique cytological or biochemical characteristics of different cell populations. Soybean agglutinin aggregates cells that bear a particular carbohydrate moiety expressed on mature blood cells, but not stem cells, allowing enrichment of the stem cells. Lymphocytes possess the CD2 antigen that acts as a receptor for sheep red blood cells. When sheep red blood cells are added to the cell mixture, the lymphocytes form rosettes around the sheep red blood cells and are then separated via differential centrifugation. Some applications take advantage of the ability of certain cell populations to adhere to the surface of specific solid substrates such as tissue-culture plastic, collagen-coated materials, and natural and synthetic polymeric scaffolds. The specifically bound cell type is selectively recovered onto the surface and removed from the initial cell suspension.
Monoclonal antibodies directed against specific cell-surface proteins can be used for both positive and negative cell selection. For example, a monoclonal antibody-bound cell population can be removed from the cell suspension after exposure to magnetic particles coated with anti-monoclonal antibody. The magnetic particles and their bound cells are removed from the cell suspension magnetically. Unlabeled cell suspensions can be poured over or incubated on surfaces such as plastic flasks or microspheres coated with monoclonal antibodies as a means of isolating particular cell populations. In addition, a fluorescence-activated cell sorter (FACS) can be used to separate different cell types by binding antibodies tagged with fluorescent markers to a particular cell type.
Other techniques enrich cell populations by destroying unwanted cells. For example, certain cell-bound monoclonal antibodies are able to fix and activate exogenously added complement, resulting in cell lysis. Some procedures use cytotoxic agents or mitotic inhibitors to selectively kill unwanted cells. These methods typically target cell subpopulations with high growth rates, such as tumor cells. Finally, an antibody can be conjugated to a toxic moiety, such as ricin, allowing delivery of the cytotoxic agent to the targeted cell population. Most of these procedures require several washing steps to ensure the removal of the dead cells, cell fragments, and cytotoxic agents from the final cell product.
Cell Ex Vivo Expansion and Differentiation
ex vivo expansion
A key issue for manufacturers of cell and tissue-based products is the ability to produce and deliver a therapeutically relevant dose of the required cell population to the patient. Depending on the application, the product may be a pure, homogeneous cell type, or it may be a mixture of different functional cell types. Many target cell populations are present at a low level or low purity in complex primary source tissues. In such cases, production of a therapeutic dose may be achieved only by specific enrichment and ex vivo expansion of the required cells.
Ex vivo expansion of cells may occur in suspension culture (e.g., T cells or hematopoietic stem and progenitor cells), adherent culture (e.g., mesenchymal stem cells, embryonic stem cells, induced pluripotent stem cells, neuronal stem cells, or dermal fibroblasts), or a mixture of both (e.g., bone marrow stroma expansion). Numerous technologies exist for cell culture. Cells can be propagated in tissue-culture flasks (T flasks), in roller bottles, on polymeric scaffolds, or in nonrigid, gas-permeable bags, usually inside incubator units controlled for temperature, humidity, and gas composition. Multilayered, high-capacity cell culture systems composed of tissue culture plastic, multibag systems, and bioreactors using microcarriers enable expansion, harvesting, and formulation to be carried out in a closed system. Traditional small-scale fermenter units can be used for expansion of cells in suspension culture. It is also possible to expand adherent cells in such units either by providing a surface for attachment (microcarriers, coated beads, or disks) or by adapting the cells to propagate in suspension culture. Some culture systems are specifically designed for the propagation of cells for therapeutic applications. These tend to be closed systems that use disposable bioreactor cartridges in automated processing units with direct control of temperature, gas composition, and media perfusion rate. In some cases automated software allows patient–donor tracking and documentation of culture conditions and manipulations. These features are useful in the design and implementation of QC product release testing programs and for the QA documentation of processing runs.
In adherent culture, the cells are usually harvested from the surface upon which they have expanded. Methods of release include physical agitation, enzymatic cleavage, and chelation of metal ions and competitive inhibition of adhesion or matrix molecules. As described above, consideration must be given to the source, safety, toxicology, and residual testing for any reagent used to release adherent cells during manufacturing. Some product-specific systems do not require the release of adherent cells. Cells are expanded on a biocompatible synthetic or natural scaffold that is then applied topically (for example, engineered skin substitutes), or the cells are grown inside or outside of fibers for ex vivo perfusion (for example, hepatocytes in hollow-fiber devices to treat liver disease).
In all cases standard cell culture parameters should be optimized for maximum process efficiency. Such parameters include composition of cellular source material, initial seeding density, media composition, rate of media exchange, temperature, gas composition, pH, and rate of delivery. Depending on the nature of the product, the potential effect of process parameters on the potency and function of the target cells should be defined.
Container–closure testing must be performed for all final container–closure systems. Compatibility for sterilization of the bioreactor and the scaffold should be verified, and the sterilization process must be validated for each product configuration. Leachables and extractables from product-contact materials such as bioreactors and packaging components should be quantified, and limits should be established.
In closed bioreactor systems it can be difficult to observe or sample cells. Measurement of metabolic parameters can provide a surrogate method that is amenable to validation with which to evaluate the rate of proliferation and predict when to harvest the cell product. The relationship of such parameters to the viability, potency, and function of the cell product should be well defined. Postexpansion purification and enrichment of target cells by using methods such as those described above may be required.
differentiation
Some cell therapies require lineage or functional differentiation of the source cells. For example, hematopoietic stem cell expansion processes normally result in products that contain a mixture of multipotent stem cells, lineage-committed progenitor cells, and lineage-differentiated cells. The composition of these products can be manipulated by different combinations of growth factors and cytokines during the expansion process. The inverse is true for processes in which mature cells are de-differentiated to enable them to then be recommitted to a lineage pathway (for example, chondrocytes in cartilage repair). Specific examples of ex vivo manipulation are the production of antigen-specific T cells to target various specific disease indications or derivation of therapeutic cell types from embryonic stem cells. Before release for clinical use, the resulting differentiated target cells must be fully characterized. Assessing the potential for de-differentiation of multipotent cells that have undergone differentiation may be necessary to ensure the safety of the product. Where the cells have been expanded and subsequently differentiated, karyotype analysis or in vitro transformation assays may be performed to demonstrate the cells are acceptable for clinical use.
ex vivo genetic manipulation
Genetic modification of cells ex vivo is a common processing procedure that is used to alter the pattern of gene expression in a defined population. The introduction of integrating or nonintegrating genetic materials (DNA, RNA, siRNA) is performed in order to induce the expression of new genes and products or to inhibit endogenous gene expression. Ex vivo genetic modification in autologous transplantation settings involves the manipulation of a harvested or expanded cell population from a patient and subsequent readministration of the cells to the donor. In a typical allogeneic transplant setting, a stable, genetically modified cell population that has been characterized and banked is administered to a broad patient population. In order to control graft-versus-host disease in allogeneic bone marrow transplants, selected donor T cells have been treated with lethal genes such as thymidine kinase that make the cells susceptible to gancyclovir treatment after transplant. Examples of autologous genetically modified cell therapy products include the transduction of tumor cells with cytokine or other immunomodulatory genes, lymphocytes transduced with receptors for tumor antigens, and the introduction into harvested lymphocytes of an antiviral ribozyme vector as a strategy to treat human immunodeficiency virus infection. Allogeneic cell therapy product examples include genetically modified and irradiated tumor cell lines used as tumor vaccines and encapsulated cells transfected with a gene to express a neurotrophic factor for localized therapeutic protein delivery in the central nervous system.
Ex vivo genetically modified cells are considered gene therapy. Issues associated with gene therapy products are addressed in detail in Gene Therapy Products  1047
1047 , especially the production of the vector or genetic material used to accomplish gene transfer, analytical testing strategies, patient safety, and monitoring. The manufacturing, cell processing, and process control methodologies addressed above are applicable in the procedures used for genetic manipulation. Frequently cell populations that are genetically modified are isolated and expanded or selected before the introduction of the genetic material. Specialized equipment and processes for introduction of genetic material exist and must be validated and monitored. Issues associated with cell banking and stability apply to cell lines used in allogeneic cell therapy products that are established and cryopreserved in MCBs and WCBs. Finally, issues associated with analysis and administration of the genetically modified cell population are discussed later in this chapter.
, especially the production of the vector or genetic material used to accomplish gene transfer, analytical testing strategies, patient safety, and monitoring. The manufacturing, cell processing, and process control methodologies addressed above are applicable in the procedures used for genetic manipulation. Frequently cell populations that are genetically modified are isolated and expanded or selected before the introduction of the genetic material. Specialized equipment and processes for introduction of genetic material exist and must be validated and monitored. Issues associated with cell banking and stability apply to cell lines used in allogeneic cell therapy products that are established and cryopreserved in MCBs and WCBs. Finally, issues associated with analysis and administration of the genetically modified cell population are discussed later in this chapter.
Formulation of Cell and Tissue-Based Products
Approaches for formulating cell and tissue-based products depend largely on the planned storage time for the cells before delivery to the patient. For some cell-based products, the time between completion of manufacturing and delivery to the intended recipient can be measured on the order of hours to days. Other cell-based products may be cryopreserved in order to extend their shelf life. A different approach for formulating cell and tissue-based products may involve the addition of a natural or synthetic scaffold that can facilitate handling, protecting the cells from immunological responses, and creating a specific shape that contributes to the therapeutic effect. Considerations for formulating each of these types of cell and tissue-based products are discussed below.
noncryopreserved cell and tissue-based products
Products consisting of suspensions of cells for delivery to patients within hours after the completion of manufacturing frequently are formulated in sterile, buffered solutions suitable for direct administration. For other noncryopreserved cell or tissue-based products extension of shelf life from hours to days may be possible by use of solutions that contain appropriate nutrients and antioxidants. In most cases these excipients are not intended for direct administration into patients. Consequently, the excipients may require removal before delivery to the patient (see Clinical Site Preparation and Administration). If an unapproved formulation buffer will be administered to patients, preclinical toxicology testing should be performed.
cryopreserved cell and tissue-based products
Most cell cryopreservation medium formulations are supplemented with 5% to 10% DMSO with or without hydroxyethyl starch (generally 6%) and a plasma protein such as 4% to 10% human serum albumin in a balanced salt solution. DMSO prevents dehydration by altering the increased concentration of nonpenetrating extracellular solutions during ice formation. The high molecular weight polymeric hydroxyethyl solution protects the cells from dehydration as water is incorporated into extracellular ice crystals. The use of protein often results in maximum recovery and viability of cells after thawing. Serum (5% to 90%) is sometimes used in place of specific proteins. Some cryopreservation formulations are completely free of protein.
The optimal concentration of cells for cryopreservation depends on the cell type and should be determined empirically, but it generally ranges from 106 to 107 cells per mL. The homogeneity and viability of the cell population being cryopreserved can also differ after thawing and should be carefully assessed. In situations where the final cell or tissue-based product is intended to be thawed and administered immediately, the presence of DMSO in the formulation buffer does subject the patient to an increased level of infusion-related toxicity, although this is related to the volume administered and the final concentration of the cryopreservative. Refer to section Clinical Site Preparation and Administration for additional considerations.
cells combined with biocompatible scaffolds
Many cell and tissue-therapy products are administered in combination with a biocompatible scaffold. For instance, wound healing or skin substitute products contain cells seeded on a scaffold. The biochemical and physical structure of the scaffold and the method for combining cells with the scaffold are specific to the product.
Cells can be loaded into a semipermeable membrane device for delivery. Usually the pore size of the membrane is large enough to allow the cell-secreted therapeutic factors to pass, but it is small enough to stop immunoglobulins and host cells from making contact with, destroying, or mounting an immune response to the therapeutic cells. The device can be a single hollow fiber or a semipermeable capsule with cells inside that secrete therapeutic compounds, or it can be part of a larger system of pumps and filters such as hollow-fiber modules with hepatocytes for the treatment of liver disease.
Cells can be seeded onto a three-dimensional scaffold and allowed to propagate and form a tissue-like structure. In the resulting product, the cells are oriented in a unique manner that is important for the intended use of the product (e.g., skin substitutes).
Cells can be encapsulated in a gel or cross-linkable polymer solution, and the resulting implantable structure can serve as a culture vessel, as a means to shield the cells from the host’s immune system, or as a way to mold cells into a defined shape. Some of the polymers used include alginate, hyaluronic acid, collagen, chitin, or synthetic polymers. Encapsulated pancreatic  -islet cells have been implanted in patients to treat diabetes. To treat urinary incontinence, chondrocytes have been mixed with alginate to form a structure upon injection.
-islet cells have been implanted in patients to treat diabetes. To treat urinary incontinence, chondrocytes have been mixed with alginate to form a structure upon injection.
Cells can be adhered to scaffolds of defined shape that are then implanted. Some examples include osteogenic precursor cells on scaffolds of demineralized cadaveric human bone, ceramic hydroxyapatite, ceramic hydroxyapatite–tricalcium phosphate, or biodegradable glass, which can be used in the repair of bone defects.
ANALYTICAL METHODS
General Considerations
The complexity and scope of cell-based therapies are reflected in the wide range of analytical methods that are used to establish in-process controls and final product release criteria. Quality specifications for cell and tissue products should be chosen to confirm the product’s quality, safety, and potency. Selected tests should be product specific and should have appropriate acceptance criteria to ensure that the product exhibits consistent quality parameters within acceptable levels of biological variation, loss of activity, physicochemical changes, or degradation throughout the product’s shelf life. The development and setting of specifications for cell and tissue products should follow the principles outlined in ICH Q6B Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products.
Specifications are established on the basis of thorough characterization of the product during the development phase and an understanding of the process and its capability. Characterization should include measurements of the physicochemical properties, safety, purity, process and product-related impurities, potency, viability, sterility, and quantity. Manufacturers should develop specifications for each product developed from this information by application of appropriate statistical methods. The data should include lots used in preclinical and clinical studies and should also include assay and process validation data that can be correlated to stability, safety, and efficacy assessments.
In-process controls and specifications for the product should be anchored by use of an appropriate reference standard. An autologous product may rely on a reference standard generated from processing cells or tissue from a healthy donor or from a source that supplies cells and tissues to research institutions. The reference standard ensures that the process, as measured by the release assays, does not change significantly over time, and it verifies that a test produces acceptable results, i.e. system suitability requirements are met. The reference standard is made from a lot that is produced under controlled conditions and passes all in-process and final release testing. In addition, this reference standard is subjected to an additional level of characterization that includes tests not normally performed for product release. The reference standard need not be stored at the same dose, formulation, or temperature as the final product. However, the stability of this reference standard must be determined.
Alternatively, a working standard can be used. If so, in the test it should behave like the reference standard. Changing to a new reference standard should include many tests, all of which are run side by side with the existing reference standard. The impact of any change in the properties of the new reference standard should be carefully evaluated before it is adopted. One option for a reference standard for a cell product with a short shelf life or for an autologous or patient-specific application can be a bank of normal donor cells of the appropriate cell type. This cell bank can be used to ensure that the manufacturing process is capable of making a consistent product.
In-Process Controls
Manufacturing processes should have well-defined go–no go decision criteria that are established for key in-process manufacturing steps. In-process controls are the assays or tests that are performed to ensure that the in-process material is of sufficient quality and quantity to ensure manufacture of an acceptable final product. Examples of in-process controls include:
- Enumeration and viability
- Microbiological (sterility, endotoxin, mycoplasma)
- Expression of phenotypic or genotypic markers
- Verification of morphology against visual reference standards
- Production of a desired bioactive substance
- Determination of population doublings, passage number, age of culture
- Assays of potential process impurities
- Monitoring of culture system parameters (% CO2, % relative humidity, pH, glucose, lactate, etc.)
- Functional tests such as colony forming units (CFU) and expression of cell-specific proteins.
A primary reason for establishing in-process control tests is to ensure that the correct product with specified quality and yield is obtained. A secondary reason for performing in-process tests is to gather process and product characterization data that can be useful in assessing the impact of process changes or excursions. Intermediate in-process material that fails to satisfy in-process control criteria should not be used for further manufacturing. This material can be reprocessed if there are procedures in place for such activities. The reprocessed material must satisfy the original in-process specifications, and the effect of reprocessing on other quality attributes such as stability must be defined before the material can undergo further manufacturing. If several sublots (e.g., cells harvested from different culture vessels) will be pooled for further processing, sublots that fail to satisfy specified criteria should not be included in the pool even if the pool containing these failed sublots would pass the in-process assay criteria.
During clinical process development, assays for product quality and yield should be performed after most processing steps to determine which steps are critical and which assays are most sensitive to deviations in the process. Statistical process controls and critical parameters should be used to establish limits for process validations and manufacturing investigations. Statistical sampling tools should be used to ensure a valid sample size. In-process controls should be performed for fully validated processes to ensure that the process continues to be under control. The results of these assays should be trended, and actions should be taken to correct problems as they arise.
Final Product Release Specifications
Cell-based therapies regulated as biological products must comply with applicable sections of 21 CFR 211 and 21 CFR 610 to ensure their identity, purity, potency, microbiological safety, and other essential attributes, such as viability, are met.
Because terminal sterilization is not possible for a living cell-based product, essentially all cell-based products are required to meet acceptance criteria for product tests such as sterility, mycoplasma, and endotoxin—typically, negative or no growth demonstrates sterility and the absence of mycoplasma, and products must demonstrate <5 endotoxin units (EU) per kilogram of patient body weight. In the case of intrathecal injection, the specified endotoxin limit is more stringent 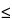 0.2 EU per kilogram of patient body weight. Adventitious virus testing is rarely performed on the final cell-based therapy product because the source cells or cell banks and ancillary materials of biological origin have been screened and tested for viral agents of concern before manufacturing.
0.2 EU per kilogram of patient body weight. Adventitious virus testing is rarely performed on the final cell-based therapy product because the source cells or cell banks and ancillary materials of biological origin have been screened and tested for viral agents of concern before manufacturing.
For almost all other final product release criteria, such as those for identity, purity, and potency, the analytical procedures with methods and acceptance criteria are specific to the individual cell-based product. Table 2 provides an overview of the expected final product release tests for cell-based therapies and lists examples of approaches that are used to satisfy the testing requirements.
Table 2. Overview of Final Product Release Testing
| Release Test | Examples | Criteria |
|---|---|---|
| Sterility | USP |
Negative |
| Mycoplasma | Direct and indirect culture method (FDA Points to Consider) | Negative; not detected |
| Endotoxin | USP |
<5 EU/kg (<0.2 EU/kg intrathecal) |
| Identity | • Surface marker determination • Isoenzyme analysis • Genetic fingerprint • Morphology • Bioassay • Biochemical marker |
Product specific |
| Purity | • Percentage of viable cells • Percentage of cells expressing specific marker(s) • Limits on undesired cell types • Limits on process contaminants (e.g., serum) |
Product specific |
| Potency | • Viable cell number • Colony-formation assay • Change in expression of specific genes • Secretion of desired macromolecule • Induction of secondary effect (e.g., human leukocyte antigen (HLA)) • Evidence of metabolic activity • Evidence of cell function |
Product specific |
| Dose | • Viable cell number • Enumeration of specific cell population • Total DNA • Total protein |
Product specific |
| Others | • Appearance • Morphology • Size |
Product specific |
sterility
Cell-based products are required to comply with final product release testing requirements, including sterility. Sterility testing is also frequently performed in-process to establish microbial purity for cells that require extended culturing. Suitable sterility tests include the test described in 21 CFR 610.12 and USP general test chapter Sterility Tests  71
71 . These culture-based test methods require 14 days and thus are suitable only for cell-based therapy products that have extended shelf lives (e.g., following cryopreservation). Many cell-based therapies have short shelf lives and must be delivered to patients before the 14-day test results are available. In such situations, FDA has identified an approach that will allow the administration of the cell-based product to patients in this setting [see Guidance for FDA Reviewers and Sponsors: Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs)]:
. These culture-based test methods require 14 days and thus are suitable only for cell-based therapy products that have extended shelf lives (e.g., following cryopreservation). Many cell-based therapies have short shelf lives and must be delivered to patients before the 14-day test results are available. In such situations, FDA has identified an approach that will allow the administration of the cell-based product to patients in this setting [see Guidance for FDA Reviewers and Sponsors: Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs)]:
- In-process sterility testing on a sample taken 48 to 72 hours before final harvest or after the last refeeding of the cultures
- A rapid microbial detection test such as a Gram stain or other procedure on the final formulated product
- Sterility testing compliant with 21 CFR 610.12 on the final formulated product
Under this alternative approach, the release criteria for sterility would be based on a negative result of the Gram stain and a no-growth result from the 48- to 72-hour in-process sterility test. In the event that the 14-day sterility test is determined to be positive after the product is administered to the subject, the manufacturer is required to report the sterility failure, results of investigation of the cause, and any corrective actions as an amendment to the IND within 30 calendar days after initial receipt of the positive culture test result.
Because of concerns regarding the sensitivity of a Gram stain and the inability to obtain full sterility results for 14 days after administration to the patient, there is widespread interest in the use of rapid microbiological methods as an alternative to the 14-day culture method. This is discussed under Alternative Test Methodologies.
mycoplasma
Mycoplasma and ureaplasma are the smallest free-living microorganisms. Mycoplasma lacks a rigid cell wall and ranges in size from 0.2 to 0.3 µm. Mycoplasma can be observed as round or filamentous in cell culture using dark-field or phase-contrast microscopy. On solid agar, colonies of mycoplasma can range in diameter from approximately 15 to 300 µm, and the larger colonies are distinguished by a typical “fried egg” appearance.
Mycoplasma is ubiquitous and can be isolated from practically all mammals. Historically it has been one of the main problems in the contamination of tissue cultures. Mycoplasma tends to be fastidious and requires preformed nucleic acids supplied by media components. These components may be readily available in the cell culturing materials that are employed during manufacturing. Mycoplasma can arise from bovine or other animal-derived culture components, cell or tissue materials from asymptomatic patients, or possibly from operators who shed it during manufacturing.
Testing for mycoplasma is recommended for all raw materials derived from a human or animal source and is required as a lot-release assay for cell-based products. FDA has published a document (Points to Consider in the Characterization of Cell Lines Used to Produce Biologicals) describing in detail the accepted methods for the cultivation and isolation of mycoplasma. Methods for mycoplasma detection are also described in USP general test chapter Mycoplasma Tests  63
63 . Because the classical assay takes one month of testing to complete, alternative methods are being developed and validated for the rapid detection of mycoplasma. This is discussed under Alternative Test Methodologies.
. Because the classical assay takes one month of testing to complete, alternative methods are being developed and validated for the rapid detection of mycoplasma. This is discussed under Alternative Test Methodologies.
endotoxins
Endotoxins exert a number of biological effects on the mammalian cell membrane and can affect secretion and cytokine production, can induce fever in recipients, or can serve as powerful mitogens. Because of the possibility of wide-ranging biologic effects of endotoxins on cell and tissue culture, raw materials and components used for the manufacture of cell-based products must be assessed for the presence of endotoxin as part of the raw materials qualification process. Control of endotoxin in the manufacture of cell therapy products is an essential element of any quality control program.
The presence of endotoxins in products administered to patients is a significant safety concern. USP general test chapter Bacterial Endotoxins Test  85
85 describes a number of different methods for measurement of endotoxins, all based on the Limulus amebocyte lysate assay. This assay can be validated for a wide range of biopharmaceutical products. An important feature of the assay with respect to cell therapy products is the ability to conduct the assay before release of products that have short shelf lives.
describes a number of different methods for measurement of endotoxins, all based on the Limulus amebocyte lysate assay. This assay can be validated for a wide range of biopharmaceutical products. An important feature of the assay with respect to cell therapy products is the ability to conduct the assay before release of products that have short shelf lives.
identity
Lot-release testing for cell-based products must include an identity test. This test serves unequivocally to identify the product. The complexity of the identity test depends on the nature of the specific product and the array of products being manufactured. For example, more extensive and rigorous testing may be performed for an autologous cell therapy product at a manufacturing facility where multiple patient products are manufactured by comparison with an allogeneic cell therapy that is the only product manufactured in a facility.
Identity tests for cell-based products must be relevant to the cell type and manipulations applied during processing. Differential surface markers are frequently used to ascertain product identity. Flow-cytometric immunoassay methods are the most common means of detecting and quantifying these markers. Identification and quantitation of particular cell subsets is accomplished by multiparameter analysis, usually of size and granularity and of one or more identity markers. Other examples of identity tests include isoenzyme analyses to confirm species of origin, which would be desirable if the product consists of xenogeneic cells. Cell morphology may be used to distinguish specific cell types. There is also an increasing trend to use genetic fingerprint technologies such as short tandem repeats to establish the identity of cell lines (e.g., human embryonic stem cells used to derive therapeutic cell types).
purity
Purity methods specifically quantify the intended active product components. Impurities are either product- or process-related residual contaminants that can be detected in the final product. The requirement to test for a particular impurity for product lot release will depend on the following: (1) the demonstrated capability of the manufacture and purification process to remove or inactivate the impurity by process validation and (2) the toxicity potential or functional product impact associated with the impurity.
Examples of process-related impurities associated with cell therapy products include residual production-medium components (e.g., serum, antibiotics, or exogenous cytokines), ancillary materials used in downstream processing (e.g., nucleases or proteases), and leachables (e.g., plasticizers from tubing or culture plastic). Impurities may be bioactive (e.g., cytokines or hormones) or immunogenic (e.g., aggregates, degradation products, or animal-derived proteins). Impurities may have other deleterious effects, depending on the dose of the product.
Product-related impurities are specific to each product type. Examples include cell debris, presence of undifferentiated cells in a cell-based product that should contain specific types of differentiated therapeutic cells; unacceptable levels of nonviable cells; replication-competent cells in a cell product that should contain mitotically inactivated cells; or changes in the composition of functional cells following cryopreservation and thaw. Analytical methodologies to assess purity require quantitation or analytical separation of the intended product from its impurities. Emphasis should be placed on demonstrating the consistency of the product-impurity profile. It may be possible to validate the manufacturing process to the extent that specific lot-release testing for impurities can be limited.
Testing for impurities is often extensive during product characterization and process validation when the consistency of the manufacturing and purification process is being demonstrated. Testing for impurities as part of lot-release testing should reflect the safety risks associated with the impurity and the ability of the process to consistently remove that impurity.
potency
Potency is defined under 21 CFR 600.3(s) as “the specific ability or capacity of the product, as indicated by appropriate laboratory tests or by adequately controlled clinical data obtained through the administration of the product in the manner intended, to effect a given result.” Together with dose, potency defines the biological activity of each lot (see Dose-Defining Assays, below). The relationship between product potency measurements during development and manufacturing to clinical safety and efficacy is key to their use in batch release. Potency may be assessed by in vitro or in vivo bioassays or a combination of the two. It is not uncommon for these assays to have coefficients of variation between 30% and 50%. These assays require a well-defined, representative reference material that can be used as a positive control for the assay. The positive control serves to qualify the performance of an individual assay. Potency assay development should focus on characterizing and controlling variability. High-precision assays are effective tools in monitoring product quality. Information about potency-assay variability should be incorporated in the stability study design and the proposed statistical approach to assignment of expiration date (see Stability, below).
The types of assays that can be used to establish the potency of a cell-based product vary widely and depend on its unique characteristics and its shelf life. For some cell-based products such as hematopoietic progenitor cells, assays for product potency have been correlated with clinical efficacy. In this case, a traditional colony-forming assay that quantifies committed progenitor cells such as colony-forming unit–granulocyte-macrophage (CFU-GM) has been correlated with clinical engraftment outcomes in some studies. For other cell-based products, in vivo animal models of disease have been used to establish product potency. If the cell-based product releases a bioactive macromolecule, a potency assay could be based on units of activity released. For example, the production of insulin in response to changes in glucose levels could be the basis of a potency assay for cells that are intended to treat diabetes.
Patient-specific products such as autologous immunotherapies present a challenge in demonstrating therapeutic activity in an in vitro or in vivo assay system. Novel approaches to measuring potency, such as the correlation of clinical outcome to other characterization tests such as identity tests, may be appropriate and should be discussed with regulatory authorities early in development. For example, the ability to determine specific cell-surface identity markers by employing flow cytometry techniques or vital stains may be an acceptable measurement of potency if properly validated and correlated with clinical outcome. FDA has issued guidance that discusses the possibility of using a matrix of biological and nonbiological assays, including both qualitative and quantitative assays, to establish product potency. Information in this guidance is particularly relevant for cell-based products that have a short shelf life or complex mechanisms of action or multiple biological activities (see Guidance for Industry: Potency Tests for Cellular and Gene Therapy Products).
A validated potency assay is typically required before regulatory approval. During investigational clinical studies, regulators typically require that a well-defined assay or assays intended to establish product potency should be in place before the initiation of pivotal clinical trials. Early implementation of one or more candidate assays intended to establish product potency is strongly encouraged. Data from these candidate potency or functional assays can be particularly important when assessing proposed manufacturing changes, during technology transfer, and in determining product stability.
dose-defining assays
An assay that precisely measures the amount of the product is referred to as a dose-defining assay, and it is selected on the basis of its accuracy and precision. An assay that measures therapeutic activity of the product is referred to as a potency assay and it is designed to measure product function. This type of assay is different from a dose-defining assay. The design of the assay depends on the type of product. In the case of drugs, assays that measure the amount of active ingredient (dose) are referred to as strength assays. For these medicines, product dose can be defined as the concentration or amount of the final product administered to the patient, and it is typically measured as product mass. For cell-based products, attributes such as viable number of therapeutic cells are often used to define the dose of the product.
Cell therapy products may be dosed on the basis of enumeration of one or more cell populations. For products in the form of a homogeneous, single-cell suspension, viable cell number is the most frequently used assay. Such assays may include enumeration of all cells, total nucleated cells, or another subset of cells. Viability assays are usually based on a cell’s ability to exclude a supravital dye, such as trypan blue. Results are expressed as the number of cells that exclude the dye and are therefore considered viable. Fluorescent compounds that bind to nuclear proteins and are excluded by viable cells may be incorporated into flow-cytometric methods for simultaneous determination of viability and cell-identity markers.
Cell counting may be performed rapidly by manual or automated methods. Manual cell counting by visual enumeration of cells in a hemacytometer chamber is a readily available technique with acceptable accuracy but a lower degree of precision than most automated methods. Typical instruments for automated cell counting provide reproducible enumeration of nonnucleated cells (e.g., erythrocytes and platelets) and nucleated cells and differential counting of the nucleated cells into mononuclear and polymorphonuclear leukocyte populations. Further discrimination of specific cell populations usually requires cell-surface phenotype analysis by flow cytometric or other methods (see Identity above). The proportion of a specific subpopulation of cells may be determined by FACS analysis or by flow cytometry. An example of a cell enumeration assay is the enumeration of CD34-positive (CD34+) hematopoietic progenitor cells.
For products that contain cells in a nonhomogeneous suspension, such as cells that are combined with a biomaterial (e.g., a scaffold), alternative measures have been used for cell enumeration, including total area of a cell sheet, wet weight, total protein, and total DNA. If such measures are used to determine product dose, then supplemental tests should be performed to establish relevance.
Considerations for Release Testing of Cell–Biomaterial Constructs
For some cell-based products such as cells combined with biomaterials to form combination products, it may not be feasible to directly test the cell–biomaterial construct. This is frequently the case when autologous cells are involved and the cell–biomaterial construct consists of a single unit and sampling of the construct is not feasible. In such cases, the individual components are tested before they are combined, and the final construct is not subjected to direct testing. Indirect measures such as sampling of the culture media can be employed to address regulatory requirements. The quality and stability of the formulated cell–biomaterial construct and relevance of indirect measures must be established by validation studies during product development.
Sampling Issues
As required by GMPs, product samples must be retained after release testing is completed. If rapid-release strategies are employed, manufacturers may need to retain additional samples so that product quality can be reassessed by alternative or traditional test methodologies if necessary.
Sampling for lot-release testing should be based on the potential distribution for the parameter tested. See Stability Protocol Development (below) for additional considerations. Samples from each lot should be retained in case there is a safety or quality issue with the lot. Even if the product has a very short shelf life, these retained samples can be used to detect impurities and other substances. The need for proper design of the sampling plan deserves special consideration. In such cases, process validation will assist in determining the appropriate statistically based sampling design.
Alternative Test Methodologies
As described under Final Product Release Specifications (above), cell-based therapies must undergo testing for sterility, mycoplasma, and endotoxin. Additional acceptance criteria for tests relating to identity, purity, potency, dose, and other relevant characteristics must be met before clinical use. With the exception of sterility, mycoplasma, and endotoxin, most of the test procedures and their underlying methods used to ensure that the final product meets release acceptance criteria are unique to the product and can be adapted to meet the specific characteristics and applications of the cell-based product. In general, test methods should be developed based on the best available science and should be suitable for use in a GMP manufacturing environment. The assays should be robust, reliable, and capable of being validated and should provide results before release for clinical use. Validation of Compendial Procedures  1225
1225 provides basic considerations for methods validation.
provides basic considerations for methods validation.
For some cell-based therapies, the sample size and volume of material required for testing or the length of time necessary to obtain test results can consume significant amounts of the final product, or the time required for obtaining results may exceed the product’s shelf life—or both. This creates problems with the available supply of product to treat patients and in other situations precludes the possibility of obtaining results before administration to patients. This is a particular problem for the compendial sterility test as well as the FDA-recommended broth-agar culture method for mycoplasma. Consequently, both industry and regulatory authorities have shown considerable interest in facilitating the development of alternative test methods for both sterility and mycoplasma.
FDA regulations for biological products specifically address the use of equivalent methods provided they ensure that the safety, purity, potency, and effectiveness of the biological product is equal to or greater than the assurances provided by the specified method (21 CFR 610.9). Some of the available alternative test methods for sterility and mycoplasma are describe below.
The range of available technologies is broad and continues to be developed by assay designers for use in the cell therapy industry. Attributes that should be included in any review of proposed technology include accuracy for the intended purpose, speed in productivity, cost, acceptability by the scientific community and regulatory agencies, simplicity of operation, training requirements and reagents, the reputation of the vendor, technical services provided by the vendor, and, finally, utility and space requirements.
Validation of these test methods and demonstration of equivalence as described in 21 CFR 610.9 are required at the time of biologics license application (BLA) or a premarket approval (PMA) submission.
sterility
Detection platforms for alternative microbiological methods have been generally based on growth, viability, artifacts, or nucleic acids. Growth-based technologies use either biochemical or physiological measures that reflect the growth of microorganisms. Test samples are transferred to traditional or enhanced media formulations that encourage microbial proliferation, and microbial growth is detected chemically or spectrophotometrically. The primary advantage of these systems is the automated nature of the test results and recovery of microorganisms for failure investigations and other microbial characterization methodologies. FDA has published guidance for validation of growth-based rapid microbiological methods (Guidance for Industry: Validation of Growth-Based Rapid Microbiological Methods for Sterility Testing of Cellular and Gene Therapy Products, CBER, 2008). Principles of validation of alternative microbiological methods are also described in USP chapter Validation of Alternative Microbiological Methods  1223
1223 .
.
Viability-based technologies do not require cells to grow. These technologies are based on detecting the presence of individual living contaminants by vital dyes, stains, or cell-surface markers. Cells labeled by a specific fluorochrome metabolic substrate are collected on a membrane for detection.
Artifact-based technologies analyze cellular components or molecular probes that are designed specifically for a particular microbial species. For example, individual species can be characterized by unique patterns of fatty acid composition after samples of whole cells have been saponified to induce the formation of methyl esters. Other technologies use time-of-flight mass spectrometry.
Nucleic acid technologies (NAT) are based on polymerase chain reaction (PCR) DNA amplification, 16s or 23s rRNA typing, and gene sequencing. Some technologies identify microorganisms by sequencing a portion of the chromosome of an unknown organism and comparing the sequence of 16s rRNA to a database. This technology is capable of identifying fungi, mycoplasma, and bacteria, including slow growers and nonfermenters. For more details on PCR-based methods, see the USP chapter Nucleic Acid-Based Techniques—Amplification  1127
1127 .
.
mycoplasma
Compendial testing methodologies for mycoplasma are growth based in agar and broth cultures and require at least 28 days to monitor appropriately the presence of mycoplasma contamination. Because of this limitation, a number of rapid mycoplasma testing technologies have been developed based on nucleic acid amplification techniques such as PCR, as well as nonamplified nucleic acid hybridization assays, ELISA, and enzyme-based assays.
QUALITY SYSTEMS
Quality systems weave together the various aspects of manufacturing. Quality control (QC) and quality assurance (QA) programs should exert control over the manufacturing facilities, the manufacturing process, the validation efforts, and all testing of the raw materials, in-process material, bulk product, and final formulated product. Training and certification programs are central to maintaining a technically competent manufacturing staff. A documentation program should be implemented to support all manufacturing, training, validation, and quality operations. Changes to processes and procedures should follow a formal program based on well-established change control principles.
When allogeneic human cells or tissues are used as the source material for the manufacturing of cell or tissue-based product, cell or tissue donors should undergo appropriate screening and testing (see Donor Eligibility, above). In all cases the source human cells and tissue must be handled in accordance with GTP as described in 21 CFR 1271.
In addition to GTPs, cGMP as outlined by FDA in 21 CFR 210, 211, 600s (especially 21 CFR 610), and 820 apply to the manufacturing of cell and tissue-based products that are subject to premarket approval. The manufacturing facility, equipment and process, raw materials, quality systems, and trained personnel are some of the key elements of cGMP. GMPs apply throughout the clinical development to both the manufacturing process and facility. The extent of control increases as clinical development progresses, and full cGMP compliance is expected by the time Phase III clinical trial(s) begin.
Data obtained from in-process and final product release testing should be monitored. Results that are out of specification (OOS), or even those that are out of trend, must be investigated before disposition of the material. FDA’s Guidance for Industry: Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production (October 2006) provides a systematic approach for conducting an investigation. An assay result can be rejected if it can be confirmed that an error, such as an analyst error, calculation error, or equipment failure, has taken place. If the investigation concludes that results of tests of the product do not fall with specified acceptance criteria, the lot should be rejected. In some situations, especially with autologous or allogeneic patient-specific product, a product that does not meet all specifications or that has only incomplete test results may have to be administered to a patient as a life-saving measure. However, procedures must be in place to govern the communication of the OOS results to the physician or to the person responsible for making the decision to use the product and to provide instruction for any follow-up testing, patient monitoring, and communication of those results to all parties, including regulatory authorities.
As discussed earlier an effective risk-management approach at the earliest stages of product development can ensure the highest quality of a cell-based product by providing a proactive measure to identify and mitigate potential quality issues. The probability for failure in cell therapy products can arise from a number of sources including personnel errors, aseptic processing failures, equipment failures, facilities and utilities failures, cleaning, disinfection, and component and raw material failures. A proactive understanding of risk can lead to improved decision making if a quality problem arises. Effective risk management can facilitate better and more informed decisions and may provide regulators with greater assurance of a developer’s ability to deal with potential risks. Such assurance can affect the extent and level of direct regulatory oversight. Quality risk management can be integrated into key parts of the quality system such as change management, Corrective and Preventive Action (CAPA), GMPs, validation, etc., and can be used to establish meaningful specifications and Critical Process Parameters (CPPs) to ensure that the quality attributes are met.
Risk analysis is qualitative in nature. It can be achieved by using experience and process knowledge to define risk categories that can form the basis of a system of risk assessment and mitigation after the identification of manufacturing errors. For example, it is common practice to develop nonconformance or deviation risk assessment categories that can be incorporated into a nonconformance or failure investigation procedure. The categories are particularly useful if the risk assessment must be expedited to facilitate a CAPA. As an example, Risk Levels 1 through 4 are defined below and can be adopted as one means of conducting a preliminary risk assessment:
Risk Level 1: Technicality—Poses no risk to the patient and does not impact the safety and effectiveness of the product. Example: A missing signature on a batch record.
Risk Level 2: Alert—May pose a safety risk to the patient or may have a potential impact on the safety and efficacy of the product. Compliance must be re-established with appropriate justification to proceed after submission to QA for review and approval. Example: Digestion time for biopsy processing falls outside a defined range.
Risk Level 3: Do not ship/reject lot—May pose a safety risk to the patient or impair the efficacy of the product even after corrective action. Shipment is not permitted. Example: Cultures fail to demonstrate adequate cell growth.
Risk Level 4: Post-distribution Event—May pose a safety risk to the patient or a potential impact on the safety, potency, or purity of the product. The safety signal is identified after product distribution. Example: Failed sterility test occurred after distribution of product.
FACILITY DESIGN AND OPERATION CONSIDERATIONS
Manufacturing facilities for cell and tissue therapy products must be carefully designed to maintain GMP aseptic processing operations while also accommodating any unique aspects of the product. Incoming cells or tissue can have bioburden and other contaminants and may need to be received and processed in a segregated area under quarantine to avoid compromising the main facility. Also, tissue processing to obtain cells of interest may require specialized equipment and processes that should be considered during the facility design and subsequent operations. For manufacture of combination products involving biocompatible scaffolds, the facility may need to be capable of handling operations that involve chemical processing, handling, and disposal. This may place constraints on the design of the facility, especially air-handling systems in clean room environments.
Although the primary emphasis in manufacturing a cell or tissue-based product is protection of the product from inadvertent contamination, risk to the manufacturing operator must be assessed and minimized by appropriate training for handling blood-borne pathogens and the use of equipment/protective clothing. Protection of the operator and aseptic processing are complementary and include the use of certified biological safety cabinets and aseptic protective clothing consisting of gowns, gloves, sleeves, surgical masks, eye protection, and head coverings. Human tissue should be obtained under environmental conditions and controls that provide a high degree of assurance for aseptic recovery.
The degree of control required for cell and tissue processing operations depends on a number of factors, including the complexity of the aseptic manufacturing process, the primary site of manufacturing, and the final product shelf life. Manufacturing processes that involve open manipulation of cells, even in a biological safety cabinet, are at greater risk of contamination than are processes done in closed bioreactors or bag systems that use sterile connections and tube-sealing devices. Typically, ISO 7 (class 10,000) clean rooms and ISO 5 (class 100) biological safety cabinets are essential components for cell therapy manufacturing processes, especially those that involve open manipulations.
The controlled environment of a carefully designed, constructed, validated, and maintained clean room can minimize the risks of environmental contamination during aseptic processing and decrease the possibility of cross-contamination of patient-specific products. The differential pressures between classified manufacturing should comply with the September 2004 guidance document, Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice. The facility and processing areas should be monitored for air quality in a manner that provides a high level of process asepsis. For guidance in this area, see Microbiological Evaluation of Clean Rooms and Other Controlled Environments  1116
1116 .
.
Facility cleaning, component and product segregation, and sanitization procedures must be in place to avoid microbial contamination and cross-contamination between lots produced in the facility.
Cell or tissue products based on autologous cells add another level of complexity to the manufacturing facility design and operation. For autologous products one product lot is made for each person, and complete segregation during manufacturing is needed. Unlike facility designs for allogeneic products, which are based on volume scale-up to achieve maximum manufacturing efficiency, facilities for autologous products require unit scale-up (scale out), which must be considered in the design and operation of the facility. Automation can be used effectively to manage repetitive manual manipulation of cells. The initial scale of operation may not justify an upfront capital investment in automation but should be considered as manufacturing operations increase in size.
Product segregation in the facility is another key consideration for design and operation. Labeling and QA oversight are traditionally used for tracking and segregation. Techniques such as bar-coding and radio-frequency (RF) tags can be used for product tracking and segregation. For guidance in this area see 21 CFR 211.42, 211.113, 1271, and FDA’s September, 2004 Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice.
Manufacturing equipment should be robust, should provide consistent product, and should allow periodic calibration and preventive maintenance. Qualification is necessary for equipment from which critical process parameters or measurements are derived. In most cases, this includes the software that controls the equipment or system’s operation. Critical equipment such as incubators and freezers need to be fitted with alarm systems that can remotely signal failure. Additionally, critical equipment should be connected to an emergency back-up generator, and the generator should be tested periodically to verify its operational state.
CONSIDERATIONS FOR VALIDATION AND QUALIFICATION
The principles of validation recommended by ICH and FDA guidance documents and USP chapters  1225
1225 and Validation of Microbial Recovery from Pharmacopeial Articles
and Validation of Microbial Recovery from Pharmacopeial Articles  1227
1227 apply to cell or tissue-based products. Biological variation in the source cells and tissues used to create most cell or tissue-based therapies may affect validation efforts.
apply to cell or tissue-based products. Biological variation in the source cells and tissues used to create most cell or tissue-based therapies may affect validation efforts.
Validation activities should include risk assessments, training and personnel qualification, equipment and facilities qualifications, analytical methods validation, aseptic processing, the manufacturing process, and cleaning.
Process validation should take into account safety, consistency (process and step yields, clearance of impurities), robustness (operator-to-operator, day-to-day), and final product quality (identity, purity, potency). Analytical methods used to assess the process should be validated or well qualified.
Process validation for patient-specific products such as autologous cell and tissue products presents some unique issues. First, the starting materials for patient-specific products typically arise from patient or matched donor materials such as biopsy material, apheresis products, scrap tissues from surgical procedures, and cadaver organs that do not qualify for transplantation. The process may require manufacturers to accept a range in the quality and quantity of starting material and yet still produce a final product that satisfies release testing. Second, manual processing of cells and tissues exhibit a degree of inherent variability. Processing steps should be developed that successfully and consistently result in appropriate process components and final product, even if the process relies on nonstandard or variable tissue materials. Process validation should take this variability into consideration and should ensure that critical manufacturing and testing endpoints consistently meet specifications.
Aseptic process validations should be performed using microbiological media to show that the manufacturing staff can execute the procedures and produce a product free of microbial contamination. Procedures intended to maintain segregation during manufacturing should be challenged to verify that there is minimal opportunity for cross-contamination or mix ups among different patient product lots.
Depending on the variability in the source cells or tissues and the complexity of the manufacturing process, it may be necessary to manufacture more than three qualification lots to verify the consistency and the robustness of the manufacturing process. Not every manufacturing effort will be successful for autologous and patient-specific therapies. However, the success rate should be established and tracked to enable manufacturers to discover any decrease in that rate and to take actions to correct the problem. Well-characterized banked primary cells may be used in the validation of the process if the donors have a range of profiles expected for the patient population. Trending of a number of statistically acceptable product administrations can also be appropriate.
Equipment and facility cleaning validations should be performed to demonstrate the efficacy of cleaning agents on standard microbial and fungal contaminants as well as environmental contaminants isolated from the manufacturing facility. Measurement of residual cleaning agents should be addressed in equipment cleaning validations. Equipment, facilities, and electronic monitoring and control systems such as building monitoring and inventory control systems should be validated.
Analytical and manufacturing equipment and methods should be validated following the principles described in  1225
1225 in addition to guidance documents issued by ICH (see Q2 R1). Training plans should be established, and personnel qualifications should be performed. Tissue transport and product shipping validations should be performed.
in addition to guidance documents issued by ICH (see Q2 R1). Training plans should be established, and personnel qualifications should be performed. Tissue transport and product shipping validations should be performed.
CLINICAL SITE PREPARATION AND ADMINISTRATION
General Considerations
Before administration of some cell or tissue-based products, one or more product modifications or preparative steps may be required. These modifications or steps are frequently performed close to the time of administration, and, therefore, they are not under the control of the original manufacturer. The nature of these modifications is largely dictated by characteristics of the product.
Preparative steps may include thawing, washing, or filtration to remove unwanted cells or substances accumulated during storage, transfer to an infusible solution, or formulation with a vehicle or structural material such as a scaffold. In addition, patient considerations, such as the need to dose or modify the product according to the patient’s anatomical structure, weight, or blood volume may influence these steps.
At the clinical site, additional procedures and process controls must be established for all product storage intervals, transport steps, and modifications, starting with a clear definition of critical control points. Operational requirements include designation of a physical space suitable for aseptic handling, such as an ISO 5 (class 100) biological safety cabinet, trained personnel, detailed standard operating procedures, and quality oversight. The unique and irreplaceable nature of many cell or tissue-based products heightens the need for well-established procedures for clinical site preparation and administration.
Product Manipulations
Before administration of medical products that contain cells, on-site preparation may involve one or more manipulations. Typical manipulations include the following:
Change in Final Container—The manufactured product may have been stored or transported in one container and may require transfer to a different container for administration.
Change in Physical State or Temperature—A product may require thawing or warming.
Change in Solution or Suspension—A product may have to be dissolved, diluted, or suspended in a liquid.
Combination with a Biomaterial—Therapeutic cells may require combination with a scaffold material such as decellularized extracellular matrix sheets, gels, plugs, capsules, sponges, particles, or granules. In other cases, cells can be added to an existing medical device such as a hollow-fiber filtration unit before use.
Admixture or Compounding—For some cell products, mixing or compounding at the clinical site may be necessary.
Filtration or Washing—The presence of unwanted materials in the manufactured product, such as particulates, cellular debris, metabolites, or compounds remaining from previous manipulations, may require washing or filtration steps.
Sampling—Sampling of the final product before administration may be required to test the final formulation.
Clinical Site Facility Considerations
Facility requirements for performing on-site preparative steps or administration of cell therapy products depend on the products and the manipulations required. The most important determinant of facility features is the level of risk for microbial contamination associated with each step. See Pharmaceutical Compounding—Sterile Preparations  797
797 for guidance that relates the type of manipulation and levels of environmental control needed to ensure aseptic handling.
for guidance that relates the type of manipulation and levels of environmental control needed to ensure aseptic handling.
Thawing Cell-Based Products
Thawing is performed rapidly. If a small number of cells will be reinfused or transplanted, DMSO does not need to be removed from the suspension because most cell preparations can be concentrated adequately to keep the DMSO concentration within tolerable limits. DMSO use has two effects on cells after thawing: Cells may clump if damaged, and DMSO reduces cell viability in minutes. If the DMSO must be removed or cells must be concentrated for administration, the thawed cell suspension is generally serially diluted (to avoid osmotic shock) and resuspended in a protein-containing medium. Cell viability and potency may be monitored after thawing, but the information is frequently intended only to gather information rather than as a specification that must be met for clinical use of the cellular product.
Some cell therapy products require that the product be shipped fresh (i.e., not frozen). In certain situations cellular components are stored frozen but are thawed and applied to scaffolds at the manufacturing site just before shipment at ambient or refrigerator temperatures. Combination products composed of cells on a scaffold may require shipping at higher than refrigerated temperatures (i.e., room temperature) to avoid dislodging cells from the scaffold. Because the fresh product is metabolically active, the shipping container must be designed and validated to maintain the metabolic activity of the product in addition to the standard shipping validation testing. Metabolism can be slowed down by lowering the shipping temperature, but this requires reliable temperature control during shipment. Because shipping containers depend somewhat on the outside temperature, the shipping container must be validated to maintain strict temperature control in all weather conditions.
Additional Release Testing of Clinical Site-Manipulated Cell Products
Cell therapy products that undergo preparative steps or manipulations at clinical sites must be subjected to appropriate checks or tests to ensure that all quality specifications are met before release for patient administration. The nature and extent of manipulations determines whether additional release requirements or critical specifications must be added to those required immediately after initial manufacture.
Prerelease steps usually include the following:
- Physical inspection of the product, including product appearance (color, turbidity, particulates, or foreign matter), container integrity, temperature, and accuracy and convenience of labeling
- Review of process records and/or certificate of analysis
- For patient-specific products, verification of product labeling and records related to identity of the intended recipient
High-risk products (defined in  797
797 ) should undergo additional testing. For all high-risk products, assess the need for and, as appropriate, perform additional quality assays for the identity, potency, and purity of the active ingredients. For high-risk products in Category II, perform sterility and endotoxin testing.
) should undergo additional testing. For all high-risk products, assess the need for and, as appropriate, perform additional quality assays for the identity, potency, and purity of the active ingredients. For high-risk products in Category II, perform sterility and endotoxin testing.
Administration to Patients
Depending on the specific cell or tissue therapy application, patient-care staff may be required to take certain steps to prepare the patient. These steps help ensure that the product will provide the intended therapeutic outcome and help minimize the risk of adverse effects.
Determination of patient suitability for the therapy, including histocompatibility evaluation, typically occurs before the product is prepared. A patient’s clinical status can change after tissue collection (because of fever, infection, recurrence or spread of tumors, or organ dysfunction), so the patient’s general condition and suitability for therapy should be reviewed before product administration. This evaluation may include a patient history, physical examination, and laboratory studies such as blood counts and chemistries. In addition, relevant baseline physical or functional measurements, laboratory tests, or imaging studies may be obtained.
Depending on the route of administration, the patient may require preparation before treatment. For cellular therapies that require intravenous administration, patients with impaired peripheral circulation may require placement of a central venous catheter. When cells or tissues combined with structural materials are implanted into the patient, the site requires preparation. This may involve establishing surgical access to the site, removing degenerated or damaged tissue, trimming adjacent tissue to accommodate the implant, and excising tissue from a second site for anchorage or support for the implant. For instance, in the case of cell products for wound healing, the site for grafting must be free from infection and must have a well-prepared wound bed. For cells to repair cartilage defects, the site of damage needs to be prepared. Before direct therapeutic administration into an organ system (e.g., the bronchioalveolar system) or vascular network (e.g., coronary arteries), the patient may require surgical, endoscopic, or radiographically directed catheter access.
In all cases, adequate anesthesia and premedication must be carefully evaluated. For example, if DMSO will remain in a thawed, cryopreserved cellular product, the patient may be given an antihistamine before administration. Pre-administration evaluation must also include assessment of concurrent therapies that may interact with the cell or tissue-therapy product to modify its effects. Some therapies may be adjunctive to the cell or tissue therapy, such as cytokines that promote proliferation or differentiation of the infused or implanted tissue. Other commonly used drugs such as antibiotics, antineoplastics, anticoagulants, and anti-inflammatory agents must be evaluated for possible effects.
delivery of cell-based therapy to patients
Some cell or tissue therapy products are patient specific because they are manufactured from a selected autologous or allogeneic tissue source, cells, or tissue. Certain patient-specific products have a defined potential for benefit or adverse immunoreactivity. Systems must be in place to prevent administration of such a product to the wrong patient. Recommended systems include procedures similar to those used for administration of human blood products, and at least two people should verify the identity of the patient and patient-specific product immediately before administration.
Cell and tissue therapy products can be administered by a variety of routes, including the common parenteral routes (intravenous, subcutaneous, intramuscular, and intra-arterial) and the respiratory or gastrointestinal tract. Other possibilities include direct application into regional vasculature, organs, tissues, or body cavities by means of needles or catheters or following surgical exposure of the tissue. Although parenteral administration can be accomplished in routine outpatient or inpatient facilities, the other means of administration may require specialized facilities such as an aseptic operating theater or endoscopic suite. A variety of delivery systems such as catheters, syringes, and IV lines are frequently used to administer cells to patients. Before clinical use manufacturers should ensure that these medical device components are compatible with the cells and formulation solutions. In all cases, standard operating procedures and a quality program must be in place to ensure that the product is administered in the intended manner.
post-administration monitoring
Written policies and procedures for monitoring patient outcomes and for reporting and managing adverse events should be in place. Patient outcome assessments should include indicators that are likely to detect errors or problems related to the entire manufacturing process, and special attention should be given to manipulations, storage, or transportation after manufacturing. Management of adverse reactions should include procedures for ensuring prompt medical evaluation and treatment of patients and a system for reporting and evaluating adverse effects that may identify potential product defects. Reporting includes information required for federal or state adverse-event monitoring programs.
STABILITY
General Considerations
The stability of cell or tissue products and the components used to create them will vary depending on the nature of the product, its intended clinical use, its specific attributes, and storage, packaging, and shipping conditions. For this reason, comprehensive guidelines covering a broad array of products are usually not possible. In all cases stability studies should be based of scientifically sound principles and a comprehensive understanding of the final therapeutic product and its intended use. Manufacturers also should assess the stability of in-process hold steps, cell banks, critical raw materials, and reference standards. A well-designed and executed stability program provides a high degree of assurance that the product is stable during its specified shelf life.
Where feasible, stability testing should be carried out in accordance with the principles described in ICH guideline Q5C, presented in Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products  1049
1049 . Stability data should also be collected for bulk and other in-process materials that are stored before final processing and filling.
. Stability data should also be collected for bulk and other in-process materials that are stored before final processing and filling.
Depending on the formulation used and storage conditions, shelf life can vary from hours to years. If a product has a short shelf life or if a product stabilizer must be removed, the final formulation may require preparation at the clinic just before administration. Instability is frequently observed as aggregation in cell products and as structural nonuniformity in tissue products. The stability of the final cell or tissue product must be established by validation studies during development.
For some cell and tissue products such as autologous or patient-specific cell products, final lots tend to have small volumes and perhaps shelf lives of only a few hours or days. In such cases, stability protocols should be based on materials from multiple donors. Because it is frequently difficult to obtain sufficient cells or tissues from autologous or patient-specific products, cells or tissues from several sources such as normal donors, research tissue repositories, cadaveric sources, or well-characterized banked primary cells can be used in stability studies to validate storage, shipping, and expiration dating. However, the results obtained with such “surrogate” cells or tissues must be interpreted cautiously and conservatively until data confirm that the actual autologous or patient-specific cells exhibit stability profiles that are similar to those of the surrogate cells or tissue.
For combination products that include cells and biomaterials, the stability of both components must be considered. When biodegradable scaffold materials are present, scaffold degradation should be considered in determining the stability and shelf life of the combination product.
Stability Protocol Development
Formal stability studies to support licensure and early-phase product stability information gathering should be detailed in a written plan that describes how stability data will be collected and analyzed to support the expiration date. Protocols should follow the format recommended in existing regulatory guidelines and should include the scope, storage conditions, and number of lots to be tested, test schedule, assays, data analysis, and product specifications. Any assay used in a formal stability study for licensure must be validated before the study begins. The specific study design should take into account the reasonably expected challenges the product may encounter. For instance, if the final product formulation is performed at the clinical site, stability studies on this final formulation should establish the time frame and conditions under which the product can be held. For cryopreserved cells or tissues, stability should be established following cryopreservation of the final product, in-process intermediates, or any hold step.
Stability studies must verify that the storage conditions maintain the quality attributes of the product so that the latter complies with stability specifications. These specifications may differ from release specifications, but they must address product potency. Measuring and calculating the decay of product activity by employing statistical methodologies may require frequent sampling during an extended period and may require analysis of multiple production lots to compensate for the variability of the assays or products.
Initial studies to establish a provisional expiration date must be conducted before administration to the first patient. Initial studies are also useful for determining which assays are stability indicating, that is, the best indicators of product degradation. Because existing compendial methods do not address the unique characteristics of all cell and tissue products, manufacturers should develop assays that address these unique characteristics.
Shipping validations are a special type of stability study with predetermined protocols, testing requirements, and acceptance criteria. Typically, the product is packaged and shipped under normal and extreme conditions, and the material is tested before and after shipping to ensure that it still meets the product release requirements. As described in Storage and Shipping (below), special attention must be given to the specific thermal, mechanical, and radiological stresses products will likely encounter.
Stability Challenge Conditions
The stability-indicating profile of a cell or tissue product may vary with time under the influence of a wide variety of environmental conditions, including temperature, mechanical stress, and light. Multifactorial degradation pathways must be considered in the development of a program to investigate the effects of these parameters on product stability. Based on risk assessment, studies should include conditions that are outside of the specified storage ranges, that is, challenge conditions such as those encountered during periods of abnormal storage, shipping, or handling. Examples include brief incubator malfunctions, incubator or cold storage failure, periods of extreme temperature fluctuation caused by shipping to hot or cold climates, hypobaric conditions in the cargo hold of a commercial airliner, or temperatures likely to be encountered in the surgical suite.
A short exposure to an environmental condition well outside of an established limit and a long exposure to one just outside of an established limit may be equally detrimental. The slow and constant rate of product degradation at a specified temperature may increase if a different set of storage conditions is applied. The effect of light on the stability-indicating profile should be investigated if it is scientifically warranted. Special attention should be given to products stored in fluids containing light-sensitive or -reactive components that may give rise to cytotoxic by-products.
Studies analogous to accelerated aging studies typically used in pharmaceutical stability-monitoring programs are also useful to characterize how the product degrades and which assays are stability indicating. For example, if a cell-based product will be held under refrigeration (4 –6
–6 ) until use, then studies performed while holding the product for extended times at room temperature (25
) until use, then studies performed while holding the product for extended times at room temperature (25 ) may provide useful information about product integrity as well as the analytical methods used. Such studies should be performed before formal stability studies begin so that the formal studies incorporate the validated stability-indicating assays into the protocol.
) may provide useful information about product integrity as well as the analytical methods used. Such studies should be performed before formal stability studies begin so that the formal studies incorporate the validated stability-indicating assays into the protocol.
STORAGE AND SHIPPING
General Considerations
Storage conditions are chosen to preserve the purity and potency of the product so that the specifications for the product are maintained throughout storage, shipping, and handling at the clinic. Before clinical trial use, initial studies must be conducted to determine acceptable storage, shipping, and handling conditions. The storage conditions and expiration date for the product must be specified. The initial storage and shipping conditions need not be exactly the same as those envisioned for the commercial product, but they should ensure that the product specifications are maintained beyond the initially proposed expiration dating. Once stability-indicating methods are developed and the final container–closure, storage, and shipping conditions are chosen, these conditions must be validated, as discussed under Stability (above).
For products with short shelf lives, storage and shipping conditions—even within a single medical center—should be considered together because shipping constitutes the bulk of storage time after manufacturing. The product should be placed in a lightproof, leakproof container with adequate physical support to ensure stability and prevention of leakage during typical conditions of shipment. Special consideration should be given to the ability of gas to permeate the shipping container, especially if the cell-therapy product is stored or shipped on dry ice or liquid nitrogen.
Storage
For each type of cellular therapy product, the manufacturer should establish product storage specifications and acceptable storage conditions, including temperature range or liquid nitrogen level. Storage units require a system that continuously monitors and records temperature and/or liquid nitrogen levels. This includes an alarm system to immediately notify lab personnel of unacceptable storage conditions. The stability of the product during routine storage should be monitored by a stability program (see Stability, above).
Cryopreservation is the main mode for the long-term storage of cells. Cellular products are cryopreserved using controlled-rate freezing procedures or equivalent procedures that are known to maintain viability. The temperature of products during freezing and storage should be monitored and documented according to the facility’s policies. The stability of the product under the holding conditions at the manufacturing facility and clinical site should also be validated.
The cooling rate for cell solutions during cryopreservation is important because of the mechanical and dehydration injuries resulting from the formation and growth of ice crystals. The ideal temperatures depend on the type of cells and the concentration of the cryopreservative. The optimal cooling rate for most cells is between 1 and 3
and 3 per minute. Controlled-rate freezers that can reproducibly duplicate this optimal cooling rate are critical when large numbers of vials or large volumes of cells in bags are being frozen. Once cooled to below freezing, cells should be stored at temperatures below
per minute. Controlled-rate freezers that can reproducibly duplicate this optimal cooling rate are critical when large numbers of vials or large volumes of cells in bags are being frozen. Once cooled to below freezing, cells should be stored at temperatures below  130
130 . This can be achieved with electric freezers or with liquid nitrogen.
. This can be achieved with electric freezers or with liquid nitrogen.
Storage of cells in the vapor phase of a liquid nitrogen freezer reduces the risk of cross-contamination with other material in the freezer. Freezer equipment should be validated and temperature mapped so that cells are not subjected to temperatures above  130
130 as the liquid nitrogen evaporates or during freezer opening. Some cells can be stored at
as the liquid nitrogen evaporates or during freezer opening. Some cells can be stored at  80
80 if the cells will be used within a few weeks. Cross-contamination can also be prevented by the use of sealed overwraps covering the cryobags.
if the cells will be used within a few weeks. Cross-contamination can also be prevented by the use of sealed overwraps covering the cryobags.
Many cell-based products cannot be cryopreserved. Because cells continue to metabolize during storage, their expiration period is short—on the order of hours or days. The expiration date can be extended by increasing the volume of storage medium, by adjusting the storage temperature, or by attaching a series of bags or compartments that allow the medium to be exchanged without breaching system sterility.
Storage conditions at the clinical site must also be defined and monitored. Cell-processing centers or clinics involved with bone marrow transplantation generally have liquid nitrogen freezers, but most clinical pharmacies do not. Storage temperatures and characteristics must be defined for each product. The clinical site may need to hold the product in the shipping container until the product can be administered to the patient. If thawing and administering of the cell-based product are performed at the clinic, the laboratory storing or shipping the cells must closely collaborate with the clinic because cells in a concentrated suspension may survive for only a few hours.
Shipping
Shipping containers and shipping procedures need to ensure temperatures are maintained within acceptable ranges for the duration of transportation and under conditions of actual use. These conditions include temperatures within the shipping container, extremes of temperature outside the shipping container (such as those encountered on a hot airport tarmac or in the chilly cargo hold of an airplane), and other shipping challenges (such as x-rays or mechanical vibration). Shipping studies should be conducted during product development in order to identify stresses to which the products may be subjected. Bracing and insulating materials should then be chosen and validated to provide a packaging system that will protect against extreme temperatures and mechanical stresses.
Most products are shipped by commercial shippers or courier systems. In some cases, critical products are hand-carried onto commercial aircraft. Commercial carriers must obtain special permission in order to bypass scanning by airport x-ray equipment. Special attention should be paid to shipping container labels because both biohazard and patient-specific information may be required in specific areas of the packaging. Shipping validations must be conducted under predefined protocols with predetermined acceptance criteria to ensure that the product meets quality specifications (including potency) once it reaches its final destination.
Cryopreserved cell-based products are typically shipped to medical centers on dry ice or in liquid nitrogen dry shippers. Dry shippers may be preferable because temperature is more readily maintained and monitored. Dry shippers also allow continuous monitoring of the shipper’s temperature, which can be collected and logged for up to 14 days. Dry ice and liquid nitrogen are both considered hazardous materials during shipping and must be labeled accordingly.
LABELING
Labeling of cell therapy products is regulated by FDA under 21 CFR 201, 601, 610, and 1271. For biologics, 21 CFR 610 Subpart G outlines the requirements for container and package labeling. When possible, a full label should be affixed to the product container. This includes the proper name of the product obtained from the US Adopted Names (USAN) Council; the name, address, and license number of the manufacturer; the lot number; the expiration date; for multiple-dose containers, the recommended individual dose; the statement “Rx Only”; instructions to the dispenser to provide a Medication Guide, if one is required, to each patient; and if the container is not enclosed in a package, all items required for a package label. If the label is too small to accommodate all this information, a partial label can be used, but the following information must appear on the partial label: the name expressed either as the proper or USAN name; the lot number and the name of the manufacturer; and for multiple-dose containers, the recommended individual dose. When partial labels are used, the container must be placed in a package that contains a label bearing all the items required for the package label. For containers that cannot accommodate any label, the container must be placed in a package that bears all the information required for a package label. In addition, when affixed to the container the label should not impede inspection of the contents. For products with very short shelf lives, expiration dating requires adjustment and correction for time zones to provide the user an accurate assessment of shelf life.
The package label must contain the proper or USAN name of the product, the name, address, and license number of manufacturer, the lot number, the expiration date, the preservative used and its concentration, or the words “No Preservative” if appropriate, the number of containers if more than one, the amount of product in the container, the recommended storage temperature, the words “Shake Well,” “Do Not Freeze,” or other instructions as indicated, the recommended individual dose for multi-dose containers, the route of administration, known sensitizing substances, the type and amount of antibiotics added during manufacture, inactive ingredients if they are a safety factor, the adjuvant, the source of the product when this is a factor for safe administration, minimum potency expressed in terms of official standard for potency or the statement “No U.S. Standard of Potency,” and finally the statement “Rx Only.” Regulations in 21 CFR 610.62 regard the position and prominence of the proper or USAN name in relation to a trade name.
Additional labeling requirements apply because cellular therapy products are also considered HCT/Ps in 21 CFR 1271.90. For autologous cell therapies, the manufacturer is exempt from the requirements of determining donor eligibility. However, if the recommended testing for pathogenic or microbial contaminants is not performed before release, the label must contain the statement “FOR AUTOLOGOUS USE ONLY” or “NOT EVALUATED FOR INFECTIOUS SUBSTANCES.” The label must also contain the Biohazard legend shown in 21 CFR 1271.3(h) with the statement “WARNING: Advise patient of communicable disease risks.” For patient-specific products, the patient’s full name, initials, or a combination of these must appear on the labeling to ensure that the product will be administered to the appropriate patient.
In addition, regulations govern the content and format of labeling for human prescription drug products (including biological products), otherwise known as the package insert. These regulations, which apply to approved therapeutics, went into effect 30 June 2006. Details about this content and format can be found in 21 CFR 201.56 and 201.57. These changes were designed to enhance the ability of the health care practitioners to access, read, and use prescription drug labeling. The main change is the addition of a half-page highlights section. Otherwise most of the changes involve rearrangement of sections to move to the front the most critical information for prescribing.
In addition to the specific FDA regulatory requirements, several groups have designed ISBT 128, a standard for uniform labeling of cellular therapy products, where ISBT stands for International Society of Blood Transfusion and the number 128 reflects the choice of barcode symbology known as Code 128. ISBT 128 defines the data structures and the placement of bar codes and their corresponding eye-readable text that appears beneath the bar code. In addition, this standard provides class names for different types of cellular products, modifier text for cell processing, manipulation text for how the cells are manufactured, cryoprotectant text for frozen cell products, and various other texts that must go on the labels. Although this voluntary standard meets different organizations’ requirements for labeling cellular products, it does not currently meet FDA regulatory requirements. Consequently, labels that comply with ISBT 128 must be supplemented with additional information required by FDA.
CONSIDERATIONS FOR TECHNOLOGY TRANSFER
Transfer of the skills, knowledge, technologies, and methods of manufacturing necessary to create a cell or tissue-based product is essential to ensure that scientific and technological developments are accessible to users who can then further develop and advance the technology into new products, processes, applications, materials, or services. Some general considerations for technology transfer activities are summarized below.
The process of developing a cellular or tissue-based therapy is complex and often involves several rounds of technology transfer throughout the product’s life cycle. Some examples of technology transfer activities include: from bench research to translational research; transfer from research and development to GMP-compliant manufacturing; and change in manufacturing facility (for example, from in-house manufacturing to a contract manufacturer).
Manufacturers should anticipate the need for technology transfer during the research and development stage of a cell or tissue-therapy process. This should result in good documentation practices for product research and development, including testing procedures. Data and results should be retained in the format of development reports or technical reports to provide historical information that can be referenced and used in regulatory filings. Critical raw materials, procedures, and equipment should also be identified during technology transfer. Product and process development progress should be monitored against milestones established as part of risk assessment and gap identification in the technology transfer plan. Table 3 provides an overview of the steps involved in technology transfer.
Table 3: Technology Transfer—Fundamental Steps
| Preparation | • Define the scope, strategy, and risks associated with the project that will be transferred • Identify overall gaps and process transferability • Assess availability of documentation such as manufacturing and testing procedures, sampling plans, in-process and final product data and specifications, material specifications (including source, testing requirements, and quantities required for a manufacturing procedure or test procedure), equipment specifications, specialized training requirements, facility requirements, and infrastructure requirements • Establish a governance body consisting of leads, experts, and mentors from both the sending and receiving sides; determine responsibilities for each group and individual • Define communication and reporting channels • Identify performance measurements, milestones, and timelines |
| Development and Implementation |
• Establish a risk management plan • Establish a technology transfer master plan • Develop a training plan • Establish documents at the receiving site (specifications, SOPs, batch records, and standard test methods) • Train operations, quality, and support personnel for sustainability • Qualify materials and vendors • Establish and execute equipment comparability/suitability protocols • Calibrate equipment at receiving site • Qualify personnel, equipment, and facility at receiving site (includes execution of aseptic process validations, sterile media fills, and cleaning validations) • Establish and execute methods/assay qualifications • Establish a product stability program • Perform engineering and consistency/qualification runs • Assess need to establish comparability and prepare regulatory filings • Establish and execute shipping qualifications |
| Maintenance | • Collect and trend process/product data • Monitor product stability • Manage change control • Train and requalify personnel • Recalibrate and requalify equipment • Update regulatory filings |
The ultimate goal of technology transfer is for the recipient to consistently reproduce a process to make comparable product in compliance with regulations. It is not atypical for manufacturers to develop and implement process improvements during early stages of technology transfer to support scale-up and manufacturing for Phase I/II clinical trials. However, during technology transfer for Phase III studies, pivotal trials, or commercial manufacturing, changes to the process or product should be avoided because they could require additional clinical studies and adversely affect time to market.
REGULATIONS AND STANDARDS
The Federal Food, Drug, and Cosmetic Act (FD&C Act) and the Public Health Service Act (PHS Act) provide the legal framework for FDA regulation of biological products, including cell-based therapy products. A list of frequently used terms in regulation of cellular-therapy products is presented in Table 4. In 1993 FDA provided notice that it intended to regulate cellular and gene-therapy products as biological products (Federal Register 1993;58:53248–53251). FDA defined somatic cell therapy products as autologous (i.e., self), allogeneic (i.e., intraspecies), or xenogeneic (i.e., interspecies) cells that have been propagated, expanded, selected, pharmacologically treated, or otherwise altered ex vivo for administration to humans for the prevention, treatment, cure, diagnosis, or mitigation of disease or injuries. For other biological products and drugs, clinical trials involving somatic cellular therapy products must be initiated under an investigational new drug (IND) application. After a sponsor submits sufficient evidence of product safety and clinical effectiveness, FDA approval can be obtained for marketing in the form of a biologics license application (BLA) or PMA.
As defined by FDA, cellular therapy products are considered to be drugs, biological products but also HCT/Ps that are regulated under Section 351 and/or Section 361 of the PHS Act. This means that cell-based therapies are subject to cGMP (21 CFR 210 and 211), Biologics Product regulations (21 CFR 610), and HCT/P regulations (21 CFR 1271) including cGTP.
In recent years FDA has issued a number of regulations and guidance documents for human cell and tissue products (see Appendix and www.fda.gov/cber/). Of particular importance are the regulations at 21 CFR 1271 that establish a tiered, risk-based approach for HCT/Ps. In this regulatory framework, many conventional human cells or tissues are not subject to premarket approval and have only to comply with GTPs, including donor eligibility. This lower tier of regulatory oversight is intended to prevent to the introduction, transmission, or spread of communicable disease. When human cells or tissue are the starting material for the creation of a novel cell-based product, additional regulatory requirements are applicable. This higher tier of regulatory oversight includes compliance with GMPs, biological product standards, and premarket approval (see 21 CFR 1271.10). In almost all cases, the cell-based products described in this general chapter should comply with the higher tier of regulatory oversight.
In addition to cellular therapy-specific regulations and guidance, many general guidelines such as those related to aseptic processing, GMP expectations during development, process validation, and others are relevant and applicable (see www.fda.gov). Additionally, ICH has issued guidance documents for qualifying cell and tissue-based products (see Appendix and www.ich.org). Some of the guidelines and concepts in these documents are reproduced in USP–NF.
The regulatory pathway for cellular-therapy products parallels that of pharmaceuticals, and as the product moves from early research through pivotal trials and finally marketing approval, the degree of manufacturing control becomes increasingly stringent. This has implications for the manufacturing unit and may dictate that the site be moved. Standards-setting organizations encourage the use of a fully functional quality unit to oversee manufacturing progress. Information is available on the FDA Web site, along with references to groups charged with guiding the medical community and the manufacturing unit during development.
In addition to USP general chapters and monographs for cell and tissue-based therapies, a number of professional standards-setting organizations (see Table 5 and Appendix) have worked closely with regulatory authorities to develop standards and practices. These organizations ensure that standards are current and comply with governmental regulations. Such standards are a supplemental source of knowledge in identification of donors, donor screening and testing, product collections, processing of cellular products, administration, adverse event reporting, and follow-up after treatment. AATB has developed guidelines for sourcing allogeneic tissue. Over the years various organizations have tried to harmonize standards, including the development of common information circulars that can be compared with package inserts. At present, however, compliance with one organization’s standards does not ensure compliance with those of any other organization.
Many benefits accrue to manufacturing facilities that participate in voluntary standards programs. Professional standards-setting organizations participate in educational workshops and disseminate information about operational issues. They also maintain close surveillance of FDA activity and training of inspectors. Further, FDA relies on accreditation by voluntary standards program, and FDA’s unannounced inspections have led to an increasingly high level of compliance in laboratory and clinical settings and has also undoubtedly increased patient safety. Third-party payors and hospital-ranking services have begun to use accreditation reports in their evaluation of quality programs.
Table 4. Frequently Used Terms in Regulation of Cellular-Therapy Products
| TERM | DEFINITION |
|---|---|
| 351 products | Regulated under Section 351 of the PHS Act |
| 361 products | Regulated under 21 CFR 1271, Human Cells, Tissues, and Cellular and Tissue-Based Products |
| BLA | Biologics Licensure Application |
| CBER | Center for Biologics Evaluation and Research |
| CDRH | Center for Devices and Radiologic Health |
| GMPs | Good Manufacturing Practices |
| GTP | Good Tissue Practices, 221 CFR 1271, Human Cells, Tissues, and Cellular and Tissue-Based Products |
| IDE | Investigational Device Exemption. An investigational device exemption (IDE) allows the investigational device to be used in a clinical study in order to collect safety and effectiveness data required to support a Premarket Approval (PA) application or a Premarket Notification [510(k)] submission to FDA. |
| IND | Investigational New Drug. An IND is a request for FDA authorization to administer an investigational drug to humans. IND regulations are contained in 21 CFR 312. |
| PMA | Premarket approval |
Table 5. Cellular Therapy Product Standards-Setting Organizations
| AABB | AABB, formerly known as the American Association of Blood Banks, is an international association representing individuals and institutions involved in activities related to transfusion and cellular therapies, including transplantation medicine. |
http://www.aabb.org/ |
| AATB | The American Association of Tissue Banks is an educational and scientific, tax-exempt organization that facilitates the provision of transplantable tissues of uniformly high quality to meet national needs. AATB publishes standards to ensure that the conduct of tissue banking meets acceptable norms of technical and ethical performance. AATB conducts an accreditation program for establishments that retrieve, process, store, or distribute human tissue for transplant. A certification program is administered for tissue-bank personnel to ensure that tissue-banking activities are performed in a profess- ional manner consistent with the standards of the association. |
http://www.aatb.org/ |
| ASTM | ASTM International (ASTM), originally known as the American Society for Testing and Materials, is one of the largest voluntary standards-development organizations in the world and provides technical standards for materials, products, systems, and services. ASTM International standards are used in the information infrastructure that guides design, manufacturing, and trade in the global economy. |
http://www.astm.org/ |
| FACT | The Foundation for the Accreditation of Cellular Therapy is a nonprofit corporation co-founded by the International Society for Cellular Therapy (ISCT) and the American Society of Blood and Marrow Transplantation (ASBMT) for voluntary inspection and accreditation in the field of cellular therapy. |
http://www.factwebsite.org/ |
| NMDP | The National Marrow Donor Program is a nonprofit organization that operates the federally funded registry of volunteer hematopoietic cell donors and umbilical cord blood units in the United States. |
http://www.nmdp.org/ |
| ICCBBA | The International Council for Commonality in Blood Banking Automation was established and given the responsibility for implementation and management of the ISBT 128 standard, a system for identification, labeling, and processing of human blood, tissue, and cellular-therapy products using an internationally standardized system. |
http://www.iccbba.org/ |
APPENDIX
Cellular therapies and cell-therapy components are regulated by FDA as biological products. The general requirements are listed in national laws and international guidance. In the United States, national requirements are codified in different sections of 21 CFR, and additional recommendations are available in FDA guidance documents. International guidance documents are available from ICH, the European Agency of Medicines (EMA), and the World Health Organization (WHO). Although guidance documents from ICH are well referenced in this general chapter, those from WHO and EMEA are not, and manufacturers of cellular or tissue-based products intended for markets outside the United States are advised to refer to relevant guidances from relevant nations. Beyond USP chapters referenced in this chapter, the following list includes regulatory documents as well as best practices in product and process development, manufacturing, quality control, and quality assurance:
Code of Federal Regulations (CFR)
- 21 CFR 201.56–57
- 21 CFR 210
- 21 CFR 211
- 21 CFR 600.3
- 21 CFR 601
- 21 CFR 610
- 21 CFR 820
- 21 CFR 1271
- 21 CFR 46
FDA Guidance Documents
- Draft Guidance for FDA Reviewers and Sponsors: Content and Review of Chemistry, Manufacturing, and Control (CMC) Information for Human Somatic Cell Therapy Investigational New Drug Applications (INDs)
- Draft Guidance for Industry: Potency Tests for Cellular and Gene Therapy Products
- Draft Guidance for Industry: Current Good Tissue Practice (cGTP) and Additional Requirements for Manufacturers of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps)
- Guidance for Industry: Eligibility Determination for Donors of Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps), February 2007. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Tissue/ucm073964.htm
- Guidance for Industry: Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans, April 2003. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/ucm074354.htm
- PHS Guideline on Infectious Disease Issues in Xenotransplantation, January 2001. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Xenotransplantation/ucm074727.htm
- Guidance for Industry: Investigating Out-of-Specification (OOS) Test Results for Pharmaceutical Production, October 2006. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM070287.pdf
- Guidance for Industry: Validation of Growth-Based Rapid Microbiological Methods for Sterility Testing of Cellular and Gene-Therapy Products, CBER, 2008. http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/CellularandGeneTherapy/ucm072612.htm
- FDA Blue Book G95-1, http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm080735.htm
National and International Regulatory Documents
- The United States Consensus Standard for the Uniform Labeling of Cellular Therapy Products using ISBT 128, available at: http://www.iccbba.org/usconsensusstandard_cellulartherapy.pdf
- ISO 10993-1:2003, Biological evaluation of medical devices—Part 1: Evaluation and testing, available at: http://www.iso.org
- ICH Q2(R1): Validation of Analytical Procedures: Text and Methodology, available at: http://www.ich.org
- ICH Q5C: Quality of Biotechnological Products: Stability Testing of Biotechnological/Biological Products, available at: http://www.ich.org
- ICH Q6B: Specifications: Test Procedures and Acceptance Criteria for Biotechnological/Biological Products, available at: http://www.ich.org
- ICH Q9: Quality Risk Management, available at: http://www.ich.org
- Naming Scheme for Cell Therapies by the United States Adopted Names (USAN) Council, available at: http://www.ama-assn.org/
- Guide for the Care and Use of Laboratory Animals (National Research Council, 1996), available at: http://www.nap.edu/
GLOSSARY AND DEFINITION OF TERMS
Adventitious Agent—
A foreign material that is introduced inadvertently; not natural or hereditary (as in microbial, chemical, or biochemical contamination of a purified substance).
Allogeneic—
From an unrelated member of the same species but with a different genotype.
Ancillary Materials—
Components used during manufacturing that should not be present in the final product. Examples: growth factors, cytokines, monoclonal antibodies, cell-separation devices, and media components.
Apheresis—
Procedure of withdrawing blood from a donor, removing select components (e.g., platelets or leukocytes), and transfusing the remainder into the donor.
Autologous—
From one’s own body.
Bioassay—
Measurement of the effectiveness of a compound by its effect on animals or cells in comparison with a standard preparation. (See also Potency.)
Biological Product—
Any virus, therapeutic serum, toxin, antitoxin, or analogous product applicable to the prevention, treatment, or cure of diseases or injuries in humans. (The term analogous product has been interpreted to include essentially all biotechnology-derived products and procedures including gene therapy, transgenics, and somatic cell therapy.)
Biotechnology—
Any technique that uses living organisms (or parts of organisms) to make or modify products, to improve plants or animals, or to develop microorganisms for specific uses. The newer definition refers to the industrial and pharmaceutical use of rDNA, cell fusion, novel bioprocessing techniques, and gene therapy.
B Lymphocytes (B Cells)—
A class of lymphocytes that produce antibodies and are derived from bone marrow.
Bone Marrow Cells—
A variety of undifferentiated cells (stem cells) and differentiated cells (lymphocytes, granulocytes, erythrocytes, and platelets) found in the internal cavities of bones or bone marrow.
Bone Marrow Transplantation—
Transplantation of bone marrow cells that are capable of maintaining the hematological functions indefinitely. Technique used in the treatment of immunological disorders (severe combined immune deficiencies such as ADA deficiency), hematological disorders (anemia), metabolic disorders (Gaucher disease), and malignant diseases (leukemia, lymphoma, or solid tumor).
CD34—
Cluster of differentiation cell-surface marker 34. CD34 is a protein that distinguishes stem and progenitor cells from more mature blood cells.
Cell Lines—
Cells that are derived from primary culture embryos, tissue, or organs. Such cell lines may have a finite life span or be immortalized (made to replicate indefinitely).
Cellular Therapy—
Therapy that uses whole cells to treat a disease, condition, or injury.
cGMP—
Current good manufacturing practice.
Chondrocytes—
Cells that produce the components of cartilage.
Clonal—
Genes, cells, or entire organisms derived from and genetically identical to a single common ancestor gene, cell, or organism.
Clonogenic Assay—
Procedure based on the ability to give rise to a clone of cells.
Combination Products—
Therapeutic products that combine drugs, devices, and/or biological products.
Cytokine—
Any factor that acts on cells; usually a protein that promotes growth.
Cytoplasm—
Cellular material that is within the cell membrane and surrounds the nucleus.
Cytotoxic—
Able to cause cell death.
Culture Medium—
The liquid that covers cells in a culture vessel and contains ingredients to nourish and support the cells. Culture medium may also include growth factors added to produce desired changes in the cells.
Dendritic Cells—
Cells that sensitize T cells to antigens.
Differentiation—
A process of biochemical and structural changes by which cells become specialized in form and function.
ELISA—
Enzyme-linked immunosorbent assay. An immunoassay that uses an enzyme-labeled antigen or antibody to detect the binding of a molecule to a solid matrix.
Embryonic Stem Cell, Human (hESC)—
Stem cell derived from the inner cell mass of the blastocyst.
Endothelial Cells—
Epithelial cells of mesodermal origin that line the internal cavities of the body, such as heart and blood and lymph vessels.
Engraftment—
Process whereby cells, tissues, or organs are implanted or transplanted into another organism. Refers both to the mechanical and the biological processes necessary to have a fully functional graft.
Epidermal—
Pertaining to the outermost and nonvascular layer of the skin derived from embryonic ectoderm.
Epithelial Cells—
Cells from the linings of various organs, e.g., respiratory, intestinal, or vascular epithelial cells.
Ex Vivo—
Outside of the living body. Refers to a medical procedure in which an organ, cells, or tissue are taken from a living body for a treatment or procedure, and then returned to the living body.
Feeder Cells—
Cells used in co-culture to maintain pluripotent stem cells. For hESC, typical feeder layers include mouse embryonic fibroblasts or human embryonic fibroblasts that have been treated to prevent them from dividing.
Fibroblasts—
Connective tissue cells that have the capacity to produce collagen.
Fluorescence-Activated Cell Sorter (FACS)—
A machine that sorts cells based on fluorescent markers attached to them.
Formulated—
Prepared in accordance with a prescribed method or conditions.
Graft-versus-Host Disease—
Rejection of the transplanted tissue by the host. It is the leading cause of patient death when mismatched allogeneic tissue is used.
Granulocyte—
One of three types of white blood cells. These cells digest bacteria and parasites.
Granulocyte–Macrophage Colony-Stimulating Factor (GM CSF)—
A natural hormone that stimulates white blood cell production, particularly that of granulocytes and monocytes.
Growth Factors—
Factors responsible for regulatory cell proliferation, function, and differentiation.
Hemacytometer—
A device used to manually count cells.
Hematopoietic—
Pertaining to or affecting the formation of blood cells.
Hematopoietic Stem Cells—
Stem cells that give rise to all red and white blood cells and platelets.
Hepatocytes—
The predominant cell type in the liver that has an important role in metabolism and is a source of serum proteins. These cells are generally not dividing, but when injured they can divide and regenerate until the injured cells are replaced.
Human Leukocyte Antigen (HLA)—
Proteins controlled by the major histocompatibility complex. These proteins play a key role in determining transplant compatibility.
Immunoassay—
Technique for identifying substances based on the use of antibodies.
Immunofluorescence—
Technique that combines an antibody detection strategy with a fluorescent label for visualization often used in combination with microscopy or fluorescence activated cell sorting.
Immunogenic—
Substance capable of inducing an immune response; a form of antigen that induces an immune response, as opposed to a tolerogen that induces tolerance.
In Vivo—
Procedure performed in the living organism.
In Vitro—
In the laboratory (outside the body). The opposite of in vivo (in the body).
Islet Cells—
 -islet cells of the pancreas that secrete insulin.
-islet cells of the pancreas that secrete insulin.
Keratinocytes—
Differentiated epidermal cells that constitute the top layer of cells in the skin.
Lineage (Committed Progenitor Cells, Differentiated Cells)—
Specific path of cell differentiation that can be traced to a single cell of origin.
Macrophage—
Any of many forms of mononuclear phagocytes that are found in tissues and arise from hematopoietic stem cells in the bone marrow.
Mesenchymal Stem Cells—
Multipotent stem cells that can differentiate into a variety of cell types.
Monoclonal Antibodies—
Antibodies that are derived from a single cell clone.
Myocytes—
Fundamental cell units in the muscle. Target cells for insertion of genes that encode secretory proteins.
Natural Killer Cells (or NK Cells)—
Cytotoxic lymphocytes that constitute a major component of the innate immune system.
Neuronal Stem Cells—
Stem cells found in neural tissue that can give rise to neurons and glial cells.
Osteogenic Cells—
Derived from or involved in the growth or repair of bone.
Passage—
The process in which cells are disassociated, washed, and seeded into new cultures after a round of cell growth and proliferation. The number of passages is a good indication of the age of the cultures and expected stability.
Process Validation—
Means for providing documentation that the manufacturing process is controlled, reproducible, and capable of consistently producing a product that meets predetermined specifications.
Polymerase Chain Reaction (PCR)—
Technique to amplify a target DNA or RNA sequence of nucleotides by cycles of polymerase-based copying, resulting in geometric increases in copy number.
Potency—
A quantitative measure of biological activity based on the attribute of the product linked to the relevant biological properties.
Progenitor Cell—
Parent or ancestral cell, usually one that is already committed to differentiate into a specific type or lineage of cells.
Regenerative Medicine—
An emerging interdisciplinary field of research and clinical applications focused on the repair, replacement or regeneration of cells, tissues or organs to restore impaired function using a combination of approaches including, but not limited to, the use of soluble molecules, gene therapy, stem cell transplantation, tissue engineering, and the reprogramming of cell and tissue types.
Serum-Free—
Refers to cell growth medium that lacks a serum component.
Somatic Cells—
Cells other than the germ cells.
Stem Cell—
Immortal cell that is capable of proliferating and differentiating into different types of specialized cells. Each major tissue system is thought to have its own putative stem cell.
Supravital Dye—
A dye that stains only living cells.
Suspension Culture—
Growth, in suspension, of cells not requiring attachment to substrate in order to undergo cell division.
T Cells—
Lymphocytes that acquire functional repertoires and the concept of self in the thymus and are responsible for cell-mediated immunity. There are several subsets of T cells (helper T cells, suppressor T cells, and cytotoxic T cells).
Umbilical Cord Blood Stem Cells—
Stem cells derived from the blood that remains in the placenta and in the attached umbilical cord after childbirth.
Undifferentiated Cells—
Cells that have not yet developed into a specialized cell type or tissue.
Xenogeneic—
From a different species.
Xenotransplantation—
Transplantation of organs from one species to another (e.g., from pigs to humans).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Fouad Atouf, Ph.D.
Senior Scientific Liaison 1-301-816-8365 |
(GCBA2010) General Chapters - Biological Analysis |
USP35–NF30 Page 502
Pharmacopeial Forum: Volume No. 36(5) Page 1289