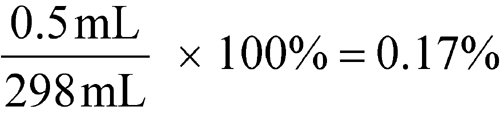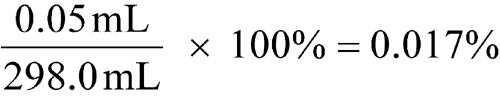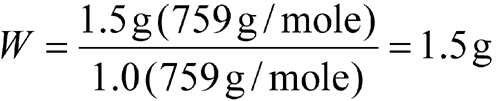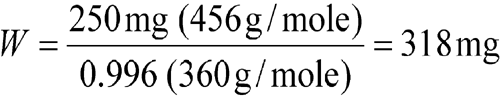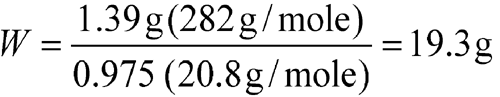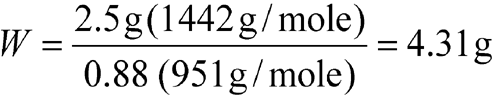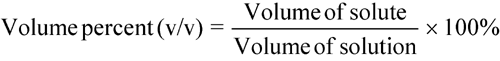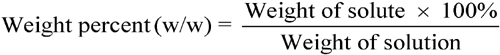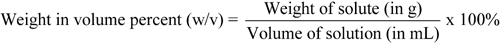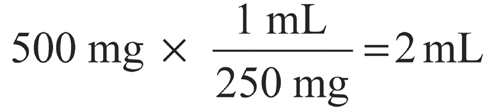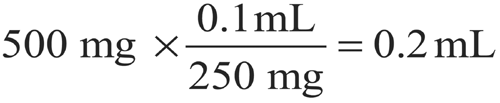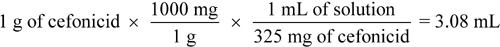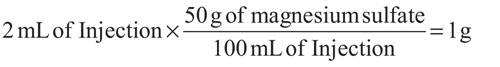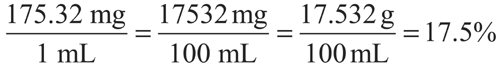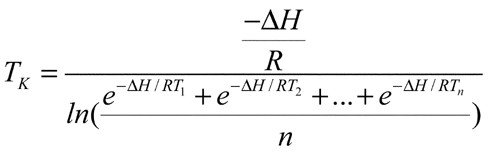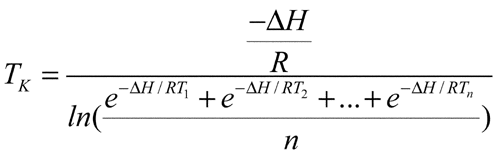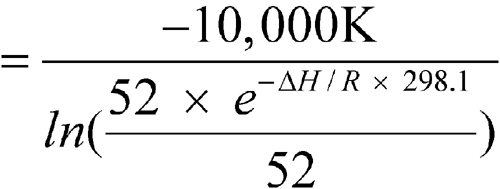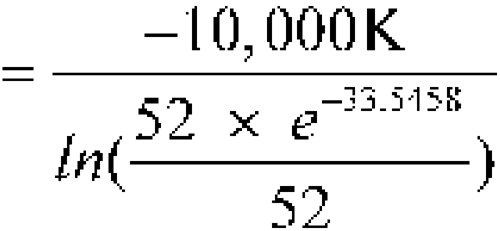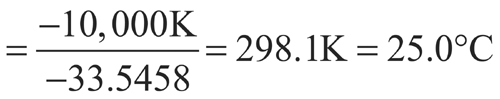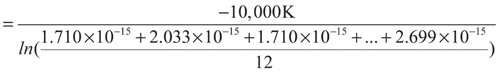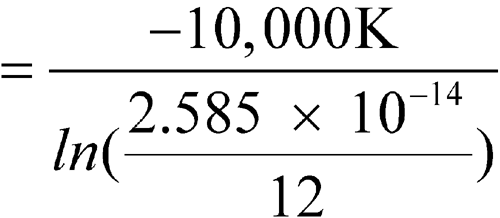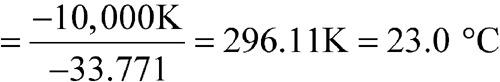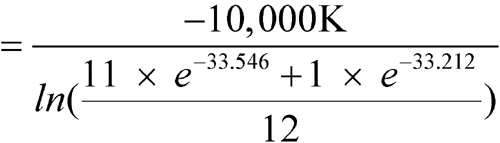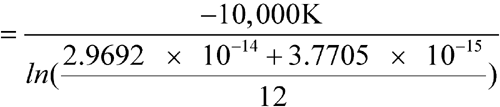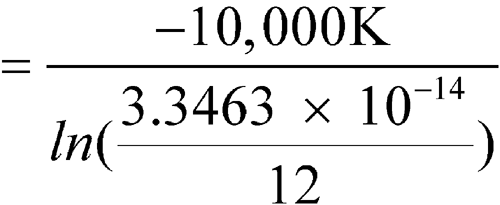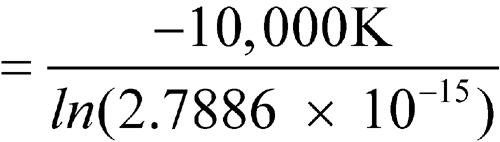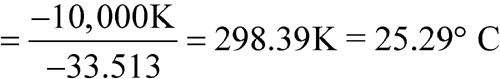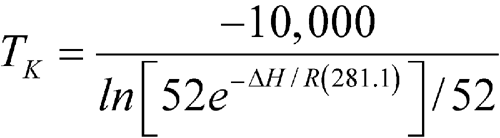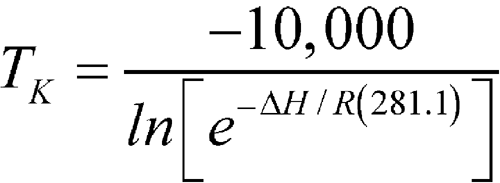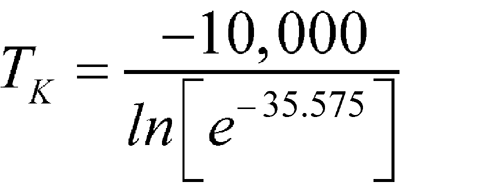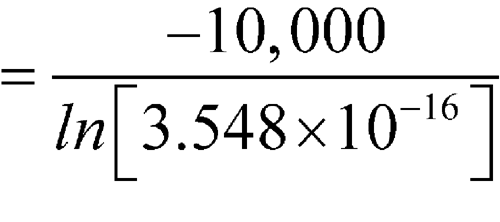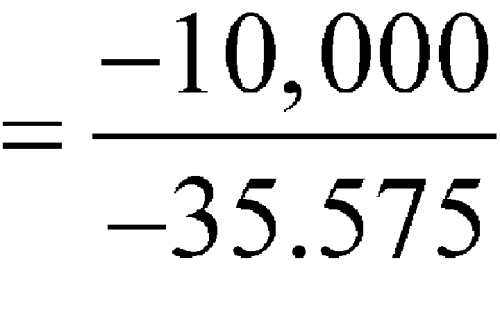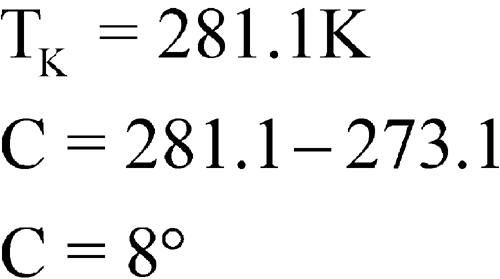INTRODUCTION
The purpose of this chapter is to provide general information to guide and assist pharmacists in performing the necessary calculations when preparing or compounding any pharmaceutical article (see Pharmaceutical Compounding—Nonsterile Preparations  795
795 , Pharmaceutical Compounding—Sterile Preparations
, Pharmaceutical Compounding—Sterile Preparations  797
797 , and Good Compounding Practices
, and Good Compounding Practices  1075
1075 ) or when simply dispensing prescriptions (see Stability Considerations in Dispensing Practice
) or when simply dispensing prescriptions (see Stability Considerations in Dispensing Practice  1191
1191 ).
).
Correct pharmaceutical calculations can be accomplished by using, for example, proper conversions from one measurement system to another and properly placed decimal points, by understanding the arithmetical concepts, and by paying close attention to the details of the calculations. Before proceeding with any calculation, pharmacists should do the following: (a) read the entire formula or prescription carefully; (b) determine which materials are needed; and then (c) select the appropriate methods of preparation and the appropriate calculation.
There are often several ways to solve a given problem. Logical methods that require as few steps as possible should be selected in order to ensure that calculations are done correctly. The best approach is the one that yields results that are accurate and free of error. The pharmacist must double-check each calculation before proceeding with the preparation of the article or prescription order. One way of double-checking is by estimation. This involves rounding off the quantities involved in the calculation, and comparing the estimated result with the calculated value.
Finally, the following steps should be taken: the dosage of each active ingredient in the prescription should be checked; all calculations should be doubly checked, preferably by another pharmacist; and where instruments are used in compounding, they should be carefully checked to ascertain that they will function properly. See USP general chapters Aerosols, Nasal Sprays, Metered-Dose Inhalers, and Dry Powder Inhalers  601
601 , Deliverable Volume
, Deliverable Volume  698
698 , Density of Solids
, Density of Solids  699
699 , Osmolality and Osmolarity
, Osmolality and Osmolarity  785
785 , pH
, pH  791
791 , Pharmaceutical Compounding—Nonsterile Preparations
, Pharmaceutical Compounding—Nonsterile Preparations  795
795 , Pharmaceutical Compounding—Sterile Preparations
, Pharmaceutical Compounding—Sterile Preparations  797
797 , Viscosity
, Viscosity  911
911 , Specific Gravity
, Specific Gravity  841
841 , Cleaning Glass Apparatus
, Cleaning Glass Apparatus  1051
1051 , Medicine Dropper
, Medicine Dropper  1101
1101 , Prescription Balances and Volumetric Apparatus
, Prescription Balances and Volumetric Apparatus  1176
1176 , Teaspoon
, Teaspoon  1221
1221 , Weighing on an Analytical Balance
, Weighing on an Analytical Balance  1251
1251 , and Good Compounding Practices
, and Good Compounding Practices  1075
1075 for information on specific instruments.
for information on specific instruments.
BASIC MATHEMATICAL CONCEPTS
significant figures
Expressed values are considered significant to the last digit shown (see Significant Figures and Tolerances in the General Notices). Significant figures are digits with practical meaning. The accuracy of the determination is implied by the number of figures used in its expression. In some calculations zeros may not be significant. For example, for a measured weight of 0.0298 g, the zeros are not significant; they are used merely to locate the decimal point. In the example, 2980 g, the zero may also be used to indicate the decimal point, in which case the zero is not significant. Alternately, however, the zero may indicate that the weight is closer to 2981 g or 2979 g, in which case the zero is significant. In such a case, knowledge of the method of measurement would be required in order to indicate whether the zero is or is not significant. In the case of a volume measurement of 298 mL, all of the digits are significant. In a given result, the last significant figure written is approximate but all preceding figures are accurate. For example, a volume of 29.8 mL implies that 8 is approximate. The true volume falls between 29.75 and 29.85. Thus, 29.8 mL is accurate to the nearest 0.1 mL, which means that the measurement has been made within ±0.05 mL. Likewise, a value of 298 mL is accurate to the nearest 1 mL and implies a measurement falling between 297.5 and 298.5, which means that the measurement has been made within ±0.5 mL and is subject to a maximum error calculated as follows:
A zero in a quantity such as 298.0 mL is a significant figure and implies that the measurement has been made within the limits of 297.95 and 298.05 with a possible error calculated as follows:
examples—
- 29.8 mL = 29.8 ± 0.05 mL (accurate to the nearest 0.1 mL)
- 29.80 mL = 29.80 ± 0.005 mL (accurate to the nearest 0.01 mL)
- 29.800 mL = 29.800 ± 0.0005 mL (accurate to the nearest 0.001 mL)
The degree of accuracy in the last example is greatest. Thus, the number of significant figures provides an estimate both of true value and of accuracy.
examples of significant figures—
| Measurement | Number of Significant Figures |
| 2.98 | 3 |
| 2.980 | 4 |
| 0.0298 | 3 |
| 0.0029 | 2 |
Calculations—
All figures should be retained until the calculations have been completed. Only the appropriate number of significant figures, however, should be retained in the final result.
Determining the number of significant figures—
Sums and Differences—
When adding or subtracting, the number of decimal places in the result shall be the same as the number of decimal places in the component with the fewest decimal places.
example—
11.5 + 11.65 + 9.90 = 33.1
Products and Quotients—
When multiplying or dividing, the result shall have no more significant figures than the measurement with the smallest number of significant figures entering into the calculation.
example—
4.266 × 21 = 90
Rounding Off—
For rules on rounding off measurements or calculated results, see Interpretation of Requirements under Significant Figures and Tolerances in the General Notices. Note, however, that in the example above, if 21 is an absolute number (e.g., the number of doses), then the answer, 89.586, is rounded off to 89.59 which has 4 significant figures.
logarithms
The logarithm of a number is the exponent or the power to which a given base must be raised in order to equal that number.
Definitions—
pH = –log [H+], and
pKa = –log Ka
pH = –log [H+], and pKa = –log Ka, where [H+] is the hydrogen ion concentration in an aqueous solution and Ka is the ionization constant of the acid in an aqueous solution. The [H+] = the antilogarithm of (–pH), and the Ka = the antilogarithm of (–pKa).
The pH of an aqueous solution containing a weak acid may be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log [salt]/[acid]
example—
A solution contains 0.020 moles per L of sodium acetate and 0.010 mole per L of acetic acid, which has a pKa value of 4.76. Calculate the pH and the [H+] of the solution. Substituting into the above equation, pH = 4.76 + log (0.020/0.010) = 5.06, and the [H+] = antilogarithm of (–5.06) = 8.69 × 10–6.
BASIC PHARMACEUTICAL CALCULATIONS
The remainder of this chapter will focus on basic pharmaceutical calculations. It is important to recognize the rules involved when adding, subtracting, dividing, and multiplying values. The interrelationships between various units within the different weighing and measuring systems are also important and have to be understood.
calculations in compounding
The pharmacist must be able to calculate the amount or concentration of drug substances in each unit or dosage portion of a compounded preparation at the time it is dispensed. Pharmacists must perform calculations and measurements to obtain, theoretically, 100% of the amount of each ingredient in compounded formulations. Calculations must account for the active ingredient, or active moiety, and water content of drug substances, which includes that in the chemical formulas of hydrates. Official drug substances and added substances must meet the requirements under Loss on Drying  731
731 , which must be included in the calculations of amounts and concentrations of ingredients. The pharmacist should consider the effect of ambient humidity on the gain or loss of water from drugs and added substances in containers subjected to intermittent opening over prolonged storage. Each container should be opened for the shortest duration necessary and then closed tightly immediately after use.
, which must be included in the calculations of amounts and concentrations of ingredients. The pharmacist should consider the effect of ambient humidity on the gain or loss of water from drugs and added substances in containers subjected to intermittent opening over prolonged storage. Each container should be opened for the shortest duration necessary and then closed tightly immediately after use.
The nature of the drug substance that is to be weighed and used in compounding a prescription must be known exactly. If the substance is a hydrate, its anhydrous equivalent weight may need to be calculated. On the other hand, if there is adsorbed moisture present that is either specified on a certificate of analysis or that is determined in the pharmacy immediately before the drug substance is used by the procedure under Loss on Drying  731
731 , this information must be used when calculating the amount of drug substance that is to be weighed in order to determine the exact amount of anhydrous drug substance required.
, this information must be used when calculating the amount of drug substance that is to be weighed in order to determine the exact amount of anhydrous drug substance required.
There are cases in which the required amount of a dose is specified in terms of a cation [e.g., Li+, netilmicin (n+)], an anion [e.g., F–], or a molecule (e.g., theophylline in aminophylline). In these instances, the drug substance weighed is a salt or complex, a portion of which represents the pharmacologically active moiety. Thus, the exact amount of such substances weighed must be calculated on the basis of the required quantity of the pharmacological moiety.
The following formula may be used to calculate the exact theoretical weight of an ingredient in a compounded preparation:
W = ab/de
in which W is the actual weighed amount; a is the prescribed or pharmacist-determined weight of the active or functional moiety of drug or added substance; b is the chemical formula weight of the ingredient, including waters of hydration for hydrous ingredients; d is the fraction of dry weight when the percent by weight of adsorbed moisture content is known from the loss on drying procedure (see Loss on Drying  731
731 ); and e is the formula weight of the active or functional moiety of a drug or added substance that is provided in the formula weight of the weighed ingredient.
); and e is the formula weight of the active or functional moiety of a drug or added substance that is provided in the formula weight of the weighed ingredient.
Example 1:
Triturate Morphine Sulfate USP and Lactose NF to obtain 10 g in which there are 30 mg of Morphine Sulfate USP for each 200 mg of the morphine-lactose mixture. [note—Clinical dosages of morphine mean Morphine Sulfate USP, which is the pentahydrate.]
| Equation Factor | Numerical Value |
| W | weight, in g, of Morphine Sulfate USP |
| a | 1.5 g of morphine sulfate pentahydrate in the prescription |
| b | 759 g/mole |
| d | 1.0 |
| e | 759 g/mole |
Example 2:
Accurately weigh an amount of Aminophylline USP to obtain 250 mg of anhydrous theophylline. [note—The powdered aminophylline dihydrate weighed contains 0.4% w/w adsorbed moisture as stated in the Certificate of Analysis.]
| Equation Factor | Numerical Value |
| W | weight, in mg, of Aminophylline USP (dihydrate) |
| a | 250 mg of theophylline |
| b | 456 g/mole |
| d | 0.996 |
| e | 360 g/mole |
Example 3:
Accurately weigh an amount of Lithium Citrate USP (containing 2.5% moisture as stated in the Certificate of Analysis) to obtain 200 mEq of lithium (Li+). [note—One mEq of Li+ is equivalent to 0.00694 g of Li+.]
| Equation Factor | Numerical Value |
| W | weight, in g, of Lithium Citrate USP (tetrahydrate) |
| a | 200 mEq of Li+ or 1.39 g of Li+ |
| b | 282 g/mole |
| d | 0.975 |
| e | 3 × 6.94 g/mole or 20.8 g/mole |
Example 4:
Accurately weigh an amount of Netilmicin Sulfate USP, equivalent to 2.5 g of netilmicin. [note—Using the procedure under Loss on Drying  731
731 , the Netilmicin Sulfate USP that was weighed lost 12% of its weight.]
, the Netilmicin Sulfate USP that was weighed lost 12% of its weight.]
| Equation Factor | Numerical Value |
| W | weight, in g, of Netilmicin Sulfate USP |
| a | 2.5 g |
| b | 1442 g/mole |
| d | 0.88 |
| e | 951 g/mole |
buffer solutions
Definition—
A buffer solution is an aqueous solution that resists a change in pH when small quantities of acid or base are added, when diluted with the solvent, or when the temperature changes. Most buffer solutions are mixtures of a weak acid and one of its salts or mixtures of a weak base and one of its salts. Water and solutions of a neutral salt such as sodium chloride have very little ability to resist the change of pH and are not capable of effective buffer action.
Preparation, Use, and Storage of Buffer Solutions—
Buffer solutions for Pharmacopeial tests should be prepared using freshly boiled and cooled water (see Standard Buffer Solutions under Buffer Solutions in Reagents, Indicators, and Solutions). They should be stored in containers such as Type I glass bottles and used within 3 months of preparation.
Buffers used in physiological systems are carefully chosen so as not to interfere with the pharmacological activity of the medicament or the normal function of the organism. Commonly used buffers in parenteral products, for example, are acetic, citric, glutamic, and phosphoric acids and their salts. Buffer solutions should be freshly prepared.
The Henderson-Hasselbalch equation, noted above, allows the pH of a buffer solution of a weak acid and its salt to be calculated. Appropriately modified, this equation may be applied to buffer solutions composed of a weak base and its salt.
Buffer Capacity—
The buffer capacity of a solution is the measurement of the ability of that solution to resist a change in pH upon addition of small quantities of a strong acid or base. An aqueous solution has a buffer capacity of 1 when 1 L of the buffer solution requires 1 gram equivalent of strong acid or base to change the pH by 1 unit. Therefore, the smaller the pH change upon the addition of a specified amount of acid or base, the greater the buffer capacity of the buffer solution. Usually, in analysis, much smaller volumes of buffer are used in order to determine the buffer capacity. An approximate formula for calculating the buffer capacity is gram equivalents of strong acid or base added per L of buffer solution per unit of pH change, i.e., (Eq /L)/(pH change).
example—
The addition of 0.01 g equivalents of sodium hydroxide to 0.25 L of a buffer solution produced a pH change of 0.50. The buffer capacity of the buffer solution is calculated as follows:
(0.01/0.25)/0.50 = 0.08(Eq/L)/(pH change)
dosage calculations
Special Dosage Regimens—
Geriatric and pediatric patients require special consideration when designing dosage regimens. In geriatric patients, the organs are often not functioning efficiently as a result of age-related pharmacokinetic changes or disease. For these patients, modifications in dosing regimens are available in references such as USP Drug Information.
For pediatric patients, where organs are often not fully developed and functioning, careful consideration must be applied during dosing. Modifications in dosing regimens for pediatric patients are also available in references such as USP Drug Information. General rules for calculating doses for infants and children are available in pharmacy calculation textbooks. These rules are not drug-specific and should be used only in the absence of more complete information.
The usual method for calculating a dose for children is to use the information provided for children for the specific drug. The dose is frequently expressed as mg of drug per kg of body weight for a 24-hour period, and is then usually given in divided portions.
The calculation may be made using the following equation:
(mg of drug per kg of body weight ) × (kg of body weight) = dose for an individual for a 24-hour period
A less frequently used method of calculating the dose is based on the surface area of the individual's body. The dose is expressed as amount of drug per body surface area in m2, as shown in the equation below:
(amount of drug per m2 of body surface area) × (body surface area in m2) = dose for an individual for a 24-hour period
The body surface area (BSA) may be determined from nomograms relating height and weight in dosage handbooks. The BSA for adult and pediatric patients may also be determined using the following equations:
BSA (m2) = square root of {[Height (in) × Weight (lb)] / 3131}
or
BSA (m2) = square root of {[Height (cm) × Weight (kg)]/ 3600}
example—
Rx for Spironolactone Suspension 25 mg/tsp. Sig: 9 mg BID for an 18 month-old child who weighs 22 lbs.
The USP DI 2002, 22nd ed., states that the normal pediatric dosing regimen for Spironolactone is 1 to 3 mg per kg per day. In this case, the weight of the child is 22 lbs, which equals 22 lbs/(2.2 lbs/kg) = 10 kg. Therefore the normal dose for this child is 10 to 30 mg per day and the dose ordered is 18 mg per day as a single dose or divided into 2 to 4 doses. The dose is acceptable based on published dosing guidelines.
percentage concentrations
Percentage concentrations of solutions are usually expressed in one of three common forms:
See also Percentage Measurements under Concentrations in the General Notices. The above three equations may be used to calculate any one of the three values (i.e., weights, volumes, or percentages) in a given equation if the other two values are known.
Note that weights are always additive, i.e., 50 g plus 25 g = 75 g. Volumes of two different solvents or volumes of solvent plus a solid solute are not strictly additive. Thus 50 mL of water + 50 mL of pure alcohol do not produce a volume of 100 mL. Nevertheless, it is assumed that in some pharmaceutical calculations, volumes are additive, as discussed below under Reconstitution of Drugs Using Volumes Other than Those on the Label.
examples—
-
Calculate the percentage concentrations (w/w) of the constituents of the solution prepared by dissolving 2.50 g of phenol in 10.00 g of glycerin. Using the weight percent equation above, the calculation is as follows.
Total weight of the solution = 10.00 g + 2.50 g = 12.50 g.Weight percent of phenol = (2.50 g × 100%)/12.50 g = 20.0% of phenol.Weight percent of glycerin = (10 g × 100%)/12.50 g = 80.0% of glycerin.
-
A prescription order reads as follows:
Eucalyptus Oil 3% (v/v) in Mineral Oil.Dispense 30.0 mL.What quantities should be used for this prescription? Using the volume percent equation above, the calculation is as follows.Amount of Eucalyptus Oil:3% = (Volume of oil in mL/30.0 mL) × 100%Solving the equation, the volume of oil = 0.90 mLAmount of Mineral Oil:To 0.90 mL of Eucalyptus Oil add sufficient Mineral Oil to prepare 30.0 mL.
-
A prescription order reads as follows:
Zinc oxide 7.5 g Calamine 7.5 g Starch 15 g White petrolatum 30 g Calculate the percentage concentration for each of the four components. Using the weight percent equation above, the calculation is as follows.Total weight = 7.5 g + 7.5 g + 15 g + 30 g = 60.0 g.Weight percent of zinc oxide = (7.5 g zinc oxide/60 g ointment) × 100% = 12.5%.Weight percent of calamine = (7.5 g calamine/60 g ointment) × 100% = 12.5%.Weight percent of starch = (15 g starch/60 g ointment) × 100% = 25%.Weight percent of white petrolatum = (30 g white petrolatum/60 g ointment) × 100% = 50%.
specific gravity
The definition of specific gravity is usually based on the ratio of weight of a substance in air at 25 to that of the weight of an equal volume of water at the same temperature. The weight of 1 mL of water at 25
to that of the weight of an equal volume of water at the same temperature. The weight of 1 mL of water at 25 is approximately 1 g. The following equation may be used for calculations.
is approximately 1 g. The following equation may be used for calculations.
Specific Gravity = (Weight of the substance)/(Weight of an equal volume of water)
examples—
-
A liquid weighs 125 g and has a volume of 110 mL. What is the specific gravity?
The weight of an equal volume of water is 110 g.Using the above equation, specific gravity = 125 g/110 g = 1.14.
-
Hydrochloric Acid NF is approximately a 37% (w/w) solution of hydrochloric acid (HCl) in water. How many grams of HCl are contained in 75.0 mL of HCl NF? (Specific gravity of Hydrochloric Acid NF is 1.18.)
Calculate the weight of HCl NF using the above equation.The weight of an equal volume of water is 75 g.Specific Gravity 1.18 = weight of the HCl NF g /75.0 g.Solving the equation, the weight of HCl NF is 88.5 g.Now calculate the weight of HCl using the weight percent equation.37.0 % (w/w) = (weight of solute g/88.5 g ) × 100.Solving the equation, the weight of the HCl is 32.7 g.
dilution and concentration
A concentrated solution can be diluted. Powders and other solid mixtures can be triturated or diluted to yield less concentrated forms. Because the amount of solute in the diluted solution or mixture is the same as the amount in the concentrated solution or mixture, the following relationship applies to dilution problems.
The quantity of Solution 1 (Q1) × concentration of Solution 1 (C1) = the quantity of Solution 2 (Q2) × concentration of Solution 2 (C2), or
(Q1)(C1) = (Q2)(C2)
Almost any quantity and concentration terms may be used. However, the units of the terms must be the same on both sides of the equation.
examples—
-
Calculate the quantity (Q2), in g, of diluent that must be added to 60 g of a 10% (w/w) ointment to make a 5% (w/w) ointment.
Let (Q1) = 60 g, (C1) = 10%, and (C2) = 5%.Using the above equation,60 g × 10% = (Q2) × 5% (w/w)Solving the above equation, the quantity of product needed, Q2, is 120 g. The initial quantity of product added was 60 g, and therefore an additional 60 g of diluent must be added to the initial quantity to give a total of 120 g.
-
How much diluent should be added to 10 g of a trituration (1 in 100) to make a mixture that contains 1 mg of drug in each 10 g of final mixture?
Determine the final concentration by first converting mg to g. One mg of drug in 10 g of mixture is the same as 0.001g in 10 g.Let (Q1) = 10 g, (C1) = (1 in 100), and (C2) = (0.001 in 10).Using the equation for dilution, 10 g × (1/100) = (Q2) g × (0.001/10).Solving the above equation, (Q2) = 1000 g.Because 10 g of the final mixture contains all of the drug and some diluent, (1000 g – 10 g) or 990 g of diluent is required to prepare the mixture at a concentration of 0.001 g of drug in 10 g of final mixture.
-
Calculate the percentage strength of a solution obtained by diluting 400 mL of a 5.0% solution to 800 mL.
Let (Q1) = 400 mL, (C1) = 5%, and (Q2) = 800 mL.Using the equation for dilution, 400 mL × 5% = 800 mL × (C2)%.Solving the above equation, (C2) = 2.5% (w/v).
use of potency units
See Units of Potency in the General Notices.
Because some substances may not be able to be defined by chemical and physical means, it may be necessary to express quantities of activity in biological units of potency.
examples—
-
One mg of Pancreatin contains not less than 25 USP Units of amylase activity, 2.0 USP Units of lipase activity, and 25 USP Units of protease activity. If the patient takes 0.1 g (100 mg) per day, what is the daily amylase activity ingested?
1 mg of Pancreatin corresponds to 25 USP Units of amylase activity.100 mg of Pancreatin corresponds to 100 × (25 USP Units of amylase activity) = 2500 Units.
-
A dose of penicillin G benzathine for streptococcal infection is 1.2 million units intramuscularly. If a specific product contains 1180 units per mg, how many milligrams would be in the dose?
1180 units of penicillin G benzathine are contained in 1 mg.1 unit is contained in 1/1180 mg.1,200,000 units are contained in (1,200,000 × 1)/1180 units = 1017 mg.
base vs salt or ester forms of drugs
Frequently, for stability or other reasons such as taste or solubility, the base form of a drug is administered in an altered form such as an ester or salt. This altered form of the drug usually has a different molecular weight (MW), and at times it may be useful to determine the amount of the base form of the drug in the altered form.
examples—
-
Four hundred milligrams of erythromycin ethylsuccinate (molecular weight, 862.1) is administered. Determine the amount of erythromycin (molecular weight, 733.9) in this dose.
862.1 g of erythromycin ethylsuccinate corresponds to 733.9 g of erythromycin.1 g of erythromycin ethylsuccinate corresponds to (733.9/862.1) g of erythromycin.0.400 g of erythromycin ethylsuccinate corresponds to (733.9/862.1) × 0.400 g or 0.3405 g of erythromycin.
-
The molecular weight of testosterone cypionate is 412.6 and that of testosterone is 288.4. What is the dose of testosterone cypionate that would be equivalent to 60.0 mg of testosterone?
288.4 g of testosterone corresponds to 412.6 g of testosterone cypionate.1 g of testosterone corresponds to 412.6/288.4 g of testosterone cypionate.60.0 mg or 0.0600 g of testosterone corresponds to (412.6/288.4) × 0.0600 = 0.0858 g or 85.8 mg of testosterone cypionate.
reconstitution of drugs using volumes other than those on the label
Occasionally it may be necessary to reconstitute a powder in order to provide a suitable drug concentration in the final product. This may be accomplished by estimating the volume of the powder and liquid medium required.
examples—
-
If the volume of 250 mg of ceftriaxone sodium is 0.1 mL, how much diluent should be added to 500 mg of ceftriaxone sodium powder to make a suspension having a concentration of 250 mg per mL?
Volume of 500 mg of ceftriaxone sodium =
Volume of the diluent required = (2 mL of suspension)
 (0.2 mL of Ceftriaxone Sodium) = 1.8 mL.
(0.2 mL of Ceftriaxone Sodium) = 1.8 mL.
-
What is the volume of dry powder cefonicid, if 2.50 mL of diluent is added to 1 g of powder to make a solution having a concentration of 325 mg per mL?
Volume of dry powder cefonicid = 3.08 mL of solution
 2.50 mL of diluent = 0.58 mL.
2.50 mL of diluent = 0.58 mL.
alligation alternate and algebra
Alligation—
Alligation is a rapid method of determining the proportions in which substances of different strengths are mixed to yield a desired strength or concentration. Once the proportion is found, the calculation may be performed to find the exact amounts of substances required. Set up the problem as follows.
- Place the desired percentage or concentration in the center.
- Place the percentage of the substance with the lower strength on the lower left-hand side.
- Place the percentage of the substance with the higher strength on the upper left-hand side.
- Subtract the desired percentage from the lower percentage, and place the obtained difference on the upper right-hand side.
- Subtract the higher percentage from the desired percentage, and place the obtained difference on the lower right-hand side.
The results obtained will determine how many parts of the two different percentage strengths should be mixed to produce the desired percentage strength of a drug mixture.
examples—
-
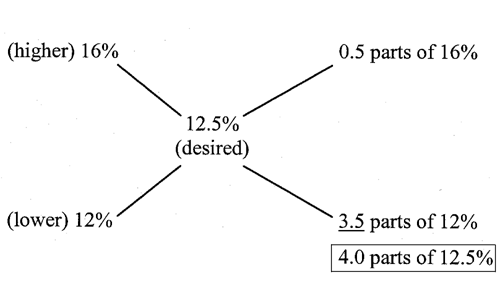
How much ointment having a 12% drug concentration and how much ointment having a 16% drug concentration must be used to make 1 kg of a preparation containing a 12.5% drug concentration?
In a total of 4.0 parts of 12.5% product, 3.5 parts of 12% ointment and 0.5 parts of 16% ointment are needed.4 parts correspond to 1 kg or 1000 g.1 part corresponds to 250 g.3.5 parts correspond to 3.5 × 250 g or 875 g.0.5 parts correspond to 0.5 × 250 g or 125 g.
-
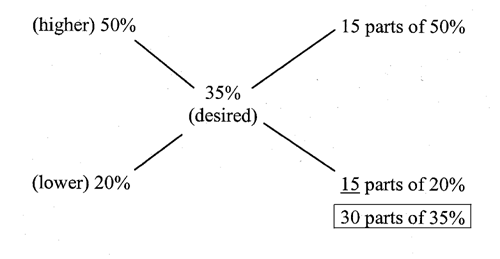
How many mL of 20% dextrose in water and 50% dextrose in water are needed to make 750 mL of 35% dextrose in water?
In a total of 30 parts of 35% dextrose in water, 15 parts of 50% dextrose in water and 15 parts of 20% dextrose in water are required.30 parts correspond to 750 mL.15 parts correspond to 375 mL.Thus use 375 mL of the 20% solution and 375 mL of the 50% solution to prepare the product.
Algebra—
Instead of using alligation to solve the above problems, algebra may be used, following the scheme outlined below.
In order to represent the total quantity (weights, parts, or volumes) of the final mixture or solution, 1 or a specified quantity is used.
Let x be the quantity of one portion and [1 (or the specified amount) – x] be the remaining portion. Set up the equation according to the statement below, and solve.
The amount of drug in one part plus the amount of drug in the other part equals the total amount in the final mixture or solution.
examples—
-
How much ointment having a 12% drug concentration and how much ointment having a 16% drug concentration must be used to make 1 kg of a preparation containing a 12.5% drug concentration?
Let 1 kg be the total quantity of ointment to be prepared, let x be the quantity, in kg, of the 12% ointment, and let (1 – x) be the quantity in kg of the 16% ointment. The equation is as follows:(12/100) x + (16/100)(1 – x) = (12.5/100)(1)Solving the equation, x equals 0.875 kg of the 12% ointment and (1 – x) equals (1 – 0.875) or 0.125 kg of the 16% ointment.
-
How many mL of 20% dextrose in water and 50% dextrose in water are needed to make 750 mL of 35% dextrose in water?
Let x be the volume, in mL, of the 20% solution, and let (750 – x) be the volume in mL of the 50% solution. The equation is as follows:(20/100)x + (50/100)(750 – x) = (35/100)(750)Solving the equation, x equals 375 mL of the 20% solution and (750 – x) equals (750 – 375) or 375 mL of the 50% solution.
molar, molal, and normal concentrations
See Concentrations in the General Notices.
Molarity—
The molar concentration, M, of the solution is the number of moles of the solute contained in one L of solution.
Molality—
The molal concentration, m, is the number of moles of the solute contained in one kilogram of solvent.
Normality—
The normal concentration, N, of a solution expresses the number of milliequivalents (mEq) of solute contained in 1 mL of solution or the number of equivalents (Eq, gram-equivalent weight) of solute contained in 1 L of solution. When using normality, the pharmacist must apply quantitative chemical analysis principles using molecular weight (MW). Normality depends on the reaction capacity of a chemical compound and therefore the reaction capacity must be known. For acids and bases, reaction capacity is the number of accessible protons available from, or the number of proton binding sites available on, each molecular aggregate. For electron transfer reactions, reaction capacity is the number of electrons gained or lost per molecular aggregate.
examples—
-
How much sodium bicarbonate powder is needed to prepare 50.0 mL of a 0.07 N solution of sodium bicarbonate (NaHCO3)? (MW of NaHCO3 is 84.0 g per mol.)
In an acid or base reaction, because NaHCO3 may act as an acid by giving up one proton, or as a base by accepting one proton, one Eq of NaHCO3 is contained in each mole of NaHCO3. Thus the equivalent weight of NaHCO3 is 84 g. [note—The volume, in L, × normality of a solution equals the number of equivalents in the solution.]The number of equivalents of NaHCO3 required = (0.07 Eq/L)(50.0 mL/1000 mL /L) = 0.0035 equivalents.1 equivalent weight is 84.0 g.0.0035 equivalents equals 84.0 g/Eq × 0.0035 Eq = 0.294 g.
-
A prescription calls for 250 mL of a 0.1 N hydrochloric acid (HCl) solution. How many mL of concentrated hydrochloric acid are needed to make this solution? [note—The specific gravity of concentrated hydrochloric acid is 1.18, the molecular weight is 36.46 and the concentration is 37.5% (w/w). Because hydrochloric acid functions as an acid and reacts by giving up one proton in a chemical reaction, 1 Eq is contained in each mole of the compound. Thus the equivalent weight is 36.46 g.]
The number of equivalents of HCl required is 0.250 L × 0.1 N = 0.025 equivalents.1 equivalent is 36.46 g.0.025 equivalents correspond to 0.025 Eq × 36.46 g/Eq = 0.9115 g.37.5 g of pure HCl are contained in 100 g of concentrated HCl.Thus 1 g of pure HCl is contained in (100/37.5) g = 2.666 g of concentrated acid, and 0.9115 g is contained in (0.9115 × 2.666) g or 2.43 g of concentrated acid.In order to determine the volume of the supplied acid required, use the definition for specific gravity as shown below.Specific gravity = (weight of the substance)/(weight of an equal volume of water).1.18 = 2.43 g/(weight of an equal volume of water).The weight of an equal volume of water is 2.056 g or 2.06 g, which measures 2.06 mL. Thus, 2.06 mL of concentrated acid is required.
milliequivalents and millimoles
note—This section addresses milliequivalents (mEq) and millimoles (mmol) as they apply to electrolytes for dosage calculations.
The quantities of electrolytes administered to patients are usually expressed in terms of mEq. This term must not be confused with a similar term used in quantitative chemical analysis as discussed above. Weight units such as mg or g are not often used for electrolytes because the electrical properties of ions are best expressed as mEq. An equivalent is the weight of a substance (equivalent weight) that supplies one unit of charge. An equivalent weight is the weight, in g, of an atom or radical divided by the valence of the atom or radical. A milliequivalent is one-thousandth of an equivalent (Eq). Because the ionization of phosphate depends on several factors, the concentration is usually expressed in millimoles, moles, or milliosmoles, which are described below. [note—Equivalent weight (Eq.wt) = wt. of an atom or radical (ion) in g/valence (or charge) of the atom or radical. Milliequivalent weight (mEq.wt) = Eq.wt. (g)/1000.]
examples—
-
Potassium (K+) has a gram-atomic weight of 39.10. The valence of K+ is 1+. Calculate its milliequivalent weight (mEq wt).
Eq wt = 39.10 g/1 = 39.10 gmEq wt = 39.10 g/1000 = 0.03910 g = 39.10 mg
-
Calcium (Ca2+) has a gram-atomic weight of 40.08. Calculate its milliequivalent weight (mEq wt).
Eq wt = 40.08 g/2 = 20.04 gmEq wt. = 20.04 g/1000 = 0.02004 g = 20.04 mgnote—The equivalent weight of a compound may be determined by dividing the molecular weight in g by the product of the valence of either relevant ion and the number of times this ion occurs in one molecule of the compound.
-
How many milliequivalents of potassium ion (K+) are there in a 250-mg Penicillin V Potassium Tablet? [note—Molecular weight of penicillin V potassium is 388.48 g per mol; there is one potassium atom in the molecule; and the valence of K+ is 1.]
Eq wt = 388.48 g/[1(valence) × 1 (number of charges)] = 388.48 g.mEq wt = 388.48 g/1000 = 0.38848 g = 388.48 mg.(250 mg per Tablet)/(388.48 mg per mEq) = 0.644 mEq of K+ per Tablet.
-
How many equivalents of magnesium ion and sulfate ion are contained in 2 mL of a 50% Magnesium Sulfate Injection? (Molecular weight of MgSO4·7H2O is 246.48 g per mol.)
Amount of magnesium sulfate in 2 mL of 50% Magnesium Sulfate InjectionEq wt of MgSO4·7H2O = MW (g)/(valence of specified ion × number of specified ions in one mole of salt).For the magnesium ion:The number of equivalents is calculated as follows:246.48/[2(valence) × 1 (number of ions in the compound)] = 123.24 g/Eq of magnesium ion.The number of equivalents in 1 g is 1g/ 123.24 g/Eq = 0.008114 Eq.The number of mEq may be calculated as follows:The mEq wt = Eq wt (g)/1000 = (123.24 g/Eq)/1000 = 0.12324 g.The number of milliequivalents of magnesium ion in 1 g is 1g/0.12324 g/mEq = 8.114 mEq.For the sulfate ion:The number of equivalents is calculated as follows:246.48/[2(valence) × 1 (number of ions in the compound)] = 123.24 g/Eq of sulfate ion.The number of equivalents in 1 g is 1g/123.24 g/Eq = 0.008114 Eq.The number of mEq may be calculated as follows:The mEq wt = Eq wt (g)/1000 = (123.24 g/Eq)/1000 = 0.12324 g.The number of milliequivalents of sulfate ion in 1 g is 1g/0.12324 g/mEq = 8.114 mEq.
-
A vial of Sodium Chloride Injection contains 3 mEq of sodium chloride per mL. What is the percentage strength of this solution? (Molecular weight of sodium chloride is 58.44 g per mol.)
1 mEq = 1 Eq/1000 = 58.44 g/1000 = 0.05844 g = 58.44 mg.Amount of sodium chloride in 3 mEq per mL = 58.44 mg per mEq × 3 mEq per mL = 175.32 mg per mL.
Using mols and mmols—
A number of countries have adopted the International System of Units and no longer calculate doses using mEq as described above, but instead use the terms moles (mol) and millimoles (mmol). In USP–NF or in the Pharmacists' Pharmacopeia the International System of Units is used except for the labeling of electrolytes.
Definitions—
A mole equals one gram atomic weight or gram molecular weight of a substance.
A millimole equals 1/1000 of a mole.
examples—
-
Potassium (K) has a gram-atomic weight of 39.10. Calculate its weight in millimoles (mmol).
The weight of one mole is 39.10 g and the weight in millimoles is 39.10 g/1000 = 0.0391 g or 39.1 mg.
-
How many millimoles of Penicillin V are in a tablet that contains 250 mg of Penicillin V Potassium? (Molecular weight of penicillin V potassium is 388.48 g per mol.)
The weight of one mole is 388.48 and the weight in millimoles is 388.48/1000 = 0.3848 g or 388.48 mg. Thus there are 250 mg/388.48 mg/mmol = 0.644 mmol of Penicillin V ion per tablet.
isoosmotic solutions
The following discussion and calculations have therapeutic implications in preparations of dosage forms intended for ophthalmic, subcutaneous, intravenous, intrathecal, and neonatal use.
Cells of the body, such as erythrocytes, will neither swell nor shrink when placed in a solution that is isotonic with the body fluids. However, the measurement of tonicity, a physiological property, is somewhat difficult. It is found that a 0.9% (w/v) solution of sodium chloride, which has a freezing point of – 0.52 , is isotonic with body fluids and is said to be isoosmotic with body fluids. In contrast to isotonicity, the freezing point depression is a physical property. Thus many solutions that are isoosmotic with body fluids are not necessarily isotonic with body fluids, e.g., a solution of urea. Nevertheless many pharmaceutical products are prepared using freezing point data or related sodium chloride data to prepare solutions that are isoosmotic with the body fluids. A closely related topic is osmolarity (see Osmolality and Osmolarity
, is isotonic with body fluids and is said to be isoosmotic with body fluids. In contrast to isotonicity, the freezing point depression is a physical property. Thus many solutions that are isoosmotic with body fluids are not necessarily isotonic with body fluids, e.g., a solution of urea. Nevertheless many pharmaceutical products are prepared using freezing point data or related sodium chloride data to prepare solutions that are isoosmotic with the body fluids. A closely related topic is osmolarity (see Osmolality and Osmolarity  785
785 ).
).
Freezing point data or sodium chloride equivalents of pharmaceuticals and excipients (see Table 1 below) may be used to prepare isoosmotic solutions, as shown in the examples below.
Table 1. Sodium Chloride Equivalents (E) and Freezing Point (FP) Depressions for a 1% Solution of the Drug or Excipient
| Drug or Excipient | E | FP Depression |
| Atropine sulfate | 0.13 | 0.075 |
| Sodium chloride | 1.00 | 0.576 |
example—
Determine the amount of sodium chloride required to prepare 60 mL of an isoosmotic solution of atropine sulfate 0.5% using the sodium chloride equivalent values and also the freezing point depression values.
Using the sodium chloride equivalent values—
The total amount of substances equivalent to sodium chloride (for a 0.9% solution) = (0.9 g/100 mL) × 60 mL = 0.54 g.
The amount of atropine sulfate required = (0.5 g/100 mL) × 60 mL = 0.3 g.
1 g of atropine sulfate is equivalent to 0.13 g of sodium chloride.
0.3 g atropine sulfate is equivalent to 0.3 × 0.13 g = 0.039 g of sodium chloride.
Thus the required amount of sodium chloride is 0.54 – 0.039 = 0.501 g or 0.50 g.
Using freezing point depression values—
The freezing point depression required is 0.52 .
.
A 1% solution of atropine sulfate causes a freezing point depression of 0.075 .
.
A 0.5% solution of atropine sulfate causes a freezing point depression of 0.075 × 0.5 = 0.0375
× 0.5 = 0.0375 .
.
The additional freezing point depression required is 0.52 – 0.0375
– 0.0375 = 0.482
= 0.482 .
.
A 1% solution of sodium chloride causes a freezing point depression of 0.576 .
.
A (1%/ 0.576) solution of sodium chloride causes a freezing point depression of 1 .
.
A (1%/ 0.576) × 0.482 = 0.836% solution of sodium chloride causes a freezing point depression of 0.482 .
.
The required amount of sodium chloride is (0.836 g/100 mL) × 60 mL = 0.502 g or 0.50 g.
flow rates in intravenous sets
Some calculations concerning flow rates in intravenous sets are provided below. [note—Examples below are not to be used for treatment purposes.]
examples—
-
Sodium Heparin 8,000 units in 250 mL Sodium Chloride Injection 0.9% solution are to be infused over 4 hours. The administration set delivers 20 drops per mL.
What is the flow rate in mL per hour?In 4 hours, 250 mL are to be delivered.In 1 hour, 250 mL/4 = 62.5 mL are delivered.What is the flow rate in drops per minute?In 60 minutes, 62.5 mL are delivered.In 1 minute, 62.5 mL/60 = 1.04 mL are delivered.1 mL = 20 drops.1.04 mL = 1.04 × 20 drops = 20.8 drops.Thus in 1 minute, 20.8 or 21 drops are administered.
-
A 14.5 kg patient is to receive 50 mg of Sodium Nitroprusside in 250 mL of dextrose 5% in water (D5W) at the rate of 1.3 µg per kg per minute. The set delivers 50 drops per mL.
Calculate the flow rate in mL per hour.The dose for 1 kg is 1.3 µg per minute.The 14.5 kg patient should receive 14.5 × 1.3 µg = 18.85 µg per minute.50 mg or 50,000 µg of drug are contained in 250 mL of D5W.18.85 µg are contained in 250 mL × 18.85/50,000 = 0.09425 mL D5W, which is administered every minute.In 1 minute, 0.09425 mL are administered.In 1 hour or 60 minutes, 60 × 0.09425 mL = 5.655 or 5.7 mL are administered.Calculate the flow rate in drops per minute.1 mL corresponds to 50 drops per minute.0.09425 mL corresponds to 0.09425 × 50 = 4.712 or 4.7 drops per minute.
temperature
The relationship between Celsius degrees ( C) and Fahrenheit degrees (
C) and Fahrenheit degrees ( F) is expressed by the following equation:
F) is expressed by the following equation:
9 ( C) = 5 (
C) = 5 ( F) – 160
F) – 160
in which  C and
C and  F are the numbers of Celsius degrees and Fahrenheit degrees, respectively.
F are the numbers of Celsius degrees and Fahrenheit degrees, respectively.
examples—
-
Convert 77
 F to Celsius degrees.
F to Celsius degrees.
9( C) = 5(
C) = 5( F) – 160
F) – 160
 C = [5(
C = [5( F) – 160]/9 = [(5 × 77) – 160]/9 = 25
F) – 160]/9 = [(5 × 77) – 160]/9 = 25  C
C
-
Convert 30
 C to Fahrenheit degrees.
C to Fahrenheit degrees.
9( C) = 5(
C) = 5( F) – 160
F) – 160
 F = [9(
F = [9( C) + 160]/5 = [(9 × 30) + 160]/5 = 86
C) + 160]/5 = [(9 × 30) + 160]/5 = 86  F
F
The relationship between the Kelvin and the Celsius scales is expressed by the equation:
K =  C + 273.1
C + 273.1
in which K and  C are the numbers of Kelvin degrees and Celsius degrees, respectively.
C are the numbers of Kelvin degrees and Celsius degrees, respectively.
application of mean kinetic temperature
See Pharmaceutical Stability  1150
1150 for the definition of mean kinetic temperature (MKT). MKT is usually higher than the arithmetic mean temperature and is derived from the Arrhenius equation. MKT addresses temperature fluctuations during the storage period of the product. The mean kinetic temperature, TK, is calculated by the following equation:
for the definition of mean kinetic temperature (MKT). MKT is usually higher than the arithmetic mean temperature and is derived from the Arrhenius equation. MKT addresses temperature fluctuations during the storage period of the product. The mean kinetic temperature, TK, is calculated by the following equation:
in which DH is the heat of activation, which equals 83.144 kJ per mol (unless more accurate information is available from experimental studies); R is the universal gas constant, which equals 8.3144 × 10–3 kJ per degree per mol; T1 is the average temperature, in degrees Kelvin, during the first time period, e.g., the first week; T2 is the average temperature, in degrees Kelvin, during the second time period, e.g., second week; and Tn is the average temperature, in degrees Kelvin during the nth time period, e.g., nth week, n being the total number of temperatures recorded. The mean kinetic temperature is calculated from average storage temperatures recorded over a one-year period, with a minimum of twelve equally spaced average storage temperature observations being recorded (see Pharmaceutical Stability  1150
1150 ). This calculation can be performed manually with a pocket calculator or electronically with computer software.
). This calculation can be performed manually with a pocket calculator or electronically with computer software.
examples—
-
The means of the highest and lowest temperatures for 52 weeks are 25
 C each. Calculate the MKT.
n = 52DH/R = 10,000 KT1, T2, ...,Tn = 25
C each. Calculate the MKT.
n = 52DH/R = 10,000 KT1, T2, ...,Tn = 25 C = 273.1 + 25 = 298.1 KR = 0.0083144 kJ K–1mol–1DH = 83.144 kJ per molThe calculated MKT is 25.0
C = 273.1 + 25 = 298.1 KR = 0.0083144 kJ K–1mol–1DH = 83.144 kJ per molThe calculated MKT is 25.0 C. Therefore the controlled room temperature requirement is met by this pharmacy. [note—If the averages of the highest and lowest weekly temperatures differed from each other and were in the allowed range of 15
C. Therefore the controlled room temperature requirement is met by this pharmacy. [note—If the averages of the highest and lowest weekly temperatures differed from each other and were in the allowed range of 15  C to 30
C to 30  C (see Controlled Room Temperature under Preservation, Packaging, Storage, and Labeling in the General Notices), then each average would be substituted individually into the equation. The remaining two examples illustrate such calculations, except that the monthly averages are used.]
C (see Controlled Room Temperature under Preservation, Packaging, Storage, and Labeling in the General Notices), then each average would be substituted individually into the equation. The remaining two examples illustrate such calculations, except that the monthly averages are used.]
-
A pharmacy recorded a yearly MKT on a monthly basis, starting in January and ending in December. Each month, the pharmacy recorded the monthly highest temperature and the monthly lowest temperature, and the average of the two was calculated and recorded for the MKT calculation at the end of the year (see Table 2). From these data the MKT may be estimated or it may be calculated. If more than half of the observed temperatures are lower than 25
 C and a mean lower than 23
C and a mean lower than 23  C is obtained, the MKT may be estimated without performing the actual calculation.
Table 2. Data for Calculation of MKT
C is obtained, the MKT may be estimated without performing the actual calculation.
Table 2. Data for Calculation of MKTn Month Lowest Temperature (in  C)
C)Highest Temperature (in  C)
C)Average Temperature (in  C)
C)Average Temperature (in K) DH/RT e–DH/RT 

1 Jan. 15 27 21 294.1 34.002 1.710 × 10–15 2 Feb. 20 25 22.5 295.6 33.830 2.033 × 10–15 3 Mar. 17 25 21 294.1 34.002 1.710 × 10–15 4 Apr. 20 25 22.5 295.6 33.830 2.033 × 10–15 5 May 22 27 24.5 297.6 33.602 2.551 × 10–15 6 June 15 25 20 293.1 34.118 1.523 × 10–15 7 July 20 26 23 296.1 33.772 2.152 × 10–15 8 Aug. 22 26 24 297.1 33.659 2.411 × 10–15 9 Sept. 23 27 25 298.1 33.546 2.699 × 10–15 10 Oct. 20 28 24 297.1 33.659 2.411 × 10–15 11 Nov. 20 24 22 295.1 33.887 1.919 × 10–15 12 Dec. 22 28 25 298.1 33.546 2.699 × 10–15 -
To estimate the MKT, the recorded temperatures are evaluated and the average is calculated. In this case, the calculated arithmetic mean is 22.9
 C. Therefore, the above requirements are met and it can be concluded that the mean kinetic temperature is lower than 25
C. Therefore, the above requirements are met and it can be concluded that the mean kinetic temperature is lower than 25  C. Therefore, the controlled room temperature requirement is met.
C. Therefore, the controlled room temperature requirement is met.
-
The second approach is to perform the actual calculation.
n = 12
The calculated MKT is 23.0 C, so the controlled room temperature requirement is met. [note—These data and calculations are used only as an example.]
C, so the controlled room temperature requirement is met. [note—These data and calculations are used only as an example.]
-
To estimate the MKT, the recorded temperatures are evaluated and the average is calculated. In this case, the calculated arithmetic mean is 22.9
-
An article was stored for one year in a pharmacy where the observed monthly average of the highest and lowest temperatures was 25
 C (298.1 K), except for one month with an average of 28
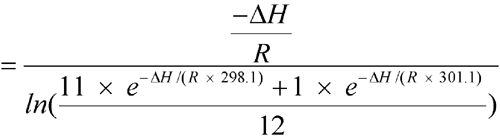
C (298.1 K), except for one month with an average of 28  C (301.1 K). Calculate the MKT of the pharmacy.
C (301.1 K). Calculate the MKT of the pharmacy.
n = 12The controlled room temperature requirement is not met because the calculated MKT exceeds 25 C. (See Note in Example 2 above.)
C. (See Note in Example 2 above.) -
Using the same calculation technique for controlled room temperature, the MKT for controlled cold temperatures can also be calculated.
-
For example, if the mean of the highest and lowest temperatures for each week over a period of 52 weeks was 8
 C (i.e., the same mean for each week), then the MKT can be calculated as follows:
C (i.e., the same mean for each week), then the MKT can be calculated as follows:
-
In another example, where a variety of average temperatures are used, as would be the case in reality, if the average of the highest and lowest temperatures ranges from 0
 to 15
to 15  C, then these averages would be individually substituted into the equation. For simplification of the mathematical process, 10 intervals are shown in Table 3 below. This illustration is intended for calculation of MKT at storage or in transit; i.e., during shipping or distribution of the critical drug product. These calculations can be performed manually or with a computer.
Table 3. Sample Data for MKT Calculations
C, then these averages would be individually substituted into the equation. For simplification of the mathematical process, 10 intervals are shown in Table 3 below. This illustration is intended for calculation of MKT at storage or in transit; i.e., during shipping or distribution of the critical drug product. These calculations can be performed manually or with a computer.
Table 3. Sample Data for MKT CalculationsIntervals Low
Temperature
(in C)
C)High
Temperature
(in C)
C)Average
Temperature
(in C)
C)Average
Temperature
(in K)DH/RT e–DH/RT × 1016 1 0 5 2.5 275.6 36.284 1.746 2 2 8 5 278.1 35.958 2.419 3 3 9 6 279.1 35.829 2.752 4 3 14 8.5 281.6 35.511 3.782 5 7 15 11 284.0 35.211 5.106 6 1 6 3.5 276.6 36.153 1.990 7 5 15 10 283.1 35.323 4.565 8 2 14 8 281.1 35.575 3.548 9 2 6 4 277.1 36.088 2.124 10 3 10 6.5 279.6 35.765 2.934
-
For example, if the mean of the highest and lowest temperatures for each week over a period of 52 weeks was 8
Auxiliary Information— Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
| General Chapter | Rick G. Schnatz
Manager, Compounding Pharmacy Expert Committee 1-301-816-8526 |
(CRX05) Compounding Pharmacy05 |
USP32–NF27 Page 674
Pharmacopeial Forum: Volume No. 31(3) Page 847