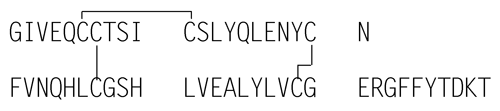Insulin Aspart
 121.1
121.1
(in' su lin as' part).
DEFINITION
Insulin Aspart is a 2-chain peptide containing 51 amino acids. The A-chain is composed of 21 amino acids, and the B-chain is composed of 30 amino acids. It is identical in primary structure to human insulin, except that it has an aspartic acid instead of proline at position 28 of the B-chain. As in human insulin, insulin aspart contains 2 interchain disulfide bonds, and 1 intrachain disulfide bond.
Insulin aspart is produced by microbial synthesis via a recombinant DNA process. The content of Insulin Aspart is NLT 90.0% and NMT 104.0% of insulin aspart (C256H381N65O79S6) plus A21Asp insulin aspart, B3Asp insulin aspart, B3isoAsp insulin aspart, and B28isoAsp insulin aspart, on the dried basis. The host cell-derived protein content is below the limit approved by the competent authority. The content of single-chain precursor is determined by a suitably sensitive method and is below the limit approved by the competent authority.
[Note—One USP Insulin Aspart Unit is equivalent to 0.0350 mg of pure insulin aspart. ]
IDENTIFICATION
• A.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
Change to read:
• B. Physicochemical Analytical Procedures for Insulins, Peptide Mapping
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 90 | 10 |
| 60 | 30 | 70 |
| 65 | 0 | 100 |
| 70 | 0 | 100 |
Chromatographic system
Mode:
LC
Detector:
UV 214 nm
Column:

 (ERR 1-Aug-2014)
(ERR 1-Aug-2014)
Column temperature:
40
Flow rate:
1 mL/min
Injection volume:
100 µL
System suitability
Sample:
Standard solution
Suitability requirements:
In the chromatogram from the Standard solution, identify the peaks due to digest fragments I, II, III, and IV. The chromatogram of the Standard solution corresponds to that of the typical chromatogram provided with USP Insulin Aspart RS.
Tailing factor:
NMT 1.5 for the peaks indicated as fragments II and III
Resolution:
NLT 8.0 between the peaks indicated as fragments II and III
Acceptance criteria:
Meets the requirements
ASSAY
• Procedure
Solution A:
Dissolve 142.0 g of anhydrous sodium sulfate in water, add 13.5 mL of phosphoric acid, and dilute with water to 5 L. Adjust, if necessary, with 10 N sodium hydroxide solution to a pH of 3.6. Mix 9 volumes of this solution with 1 volume of acetonitrile.
Solution B:
Acetonitrile and water (1:1)
Mobile phase:
See Table 2.
Table 2
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 58 | 42 |
| 35 | 58 | 42 |
| 40 | 20 | 80 |
| 45 | 20 | 80 |
| 46 | 58 | 42 |
| 60 | 58 | 42 |
[Note—Adjust the Mobile phase composition and the duration of the isocratic elution to obtain a retention time of about 22 min for the insulin aspart and to ensure that B3isoAsp insulin aspart is eluted before the gradient starts. ]
System suitability solution:
Use an appropriate solution with a content of B3Asp insulin aspart and A21Asp insulin aspart of NLT 1.0%. This may be achieved by storing the Standard solution at room temperature for about 1–3 days.
Standard solution:
Dissolve the contents of a vial of USP Insulin Aspart RS in 0.01 N hydrochloric acid to obtain a concentration of 4.0 mg/mL.
Sample solution:
Dissolve the sample to be examined in 0.01 N hydrochloric acid to obtain a concentration of 4.0 mg/mL.
Chromatographic system
Mode:
LC
Detector:
UV 214 nm
Column:
4.0-mm × 25.0-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1 mL/min
Injection volume:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for B28isoAsp insulin aspart, insulin aspart, B3Asp insulin aspart plus A21Asp insulin aspart (generally coelute), and B3isoAsp insulin aspart are about 0.9, 1.0, 1.3, and 1.5 min, respectively. ]
Suitability requirements
Relative standard deviation:
NMT 1.4% for five replicate injections, Standard solution
Resolution:
NLT 2.0 between the peak for insulin aspart and the peak for A21Asp insulin aspart and for B3Asp insulin aspart, System suitability solution
Tailing factor:
NMT 1.5 for the insulin aspart peak, System suitability solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the content of insulin aspart (C256H381N65O79S6) plus B28isoAsp insulin aspart, A21Asp insulin aspart, B3Asp insulin aspart, and B3isoAsp insulin aspart in percentage using the areas of the corresponding peaks in the chromatograms from the Sample solution and the Standard solution and the declared content of insulin aspart plus B28isoAsp insulin aspart, A21Asp insulin aspart, B3Asp insulin aspart, and B3isoAsp insulin aspart in the USP Insulin Aspart RS taken:
Result = (rIA(U) + rB28iso(U) + rA21(U) + rB3(U) + rB3iso(U))/(rIA(S) + rB28iso(S) + rA21(S) + rB3(S) + rB3iso(S)) × (CS/CU) × 100
| rIA(U) | = | = peak response for insulin aspart from the Sample solution |
| rB28iso(U) | = | = peak response for B28isoAsp insulin aspart from the Sample solution |
| rA21(U) | = | = peak response for A21Asp insulin aspart from the Sample solution |
| rB3(U) | = | = peak response for B3Asp insulin aspart from the Sample solution |
| rB3iso(U) | = | = peak response for B3isoAsp insulin aspart from the Sample solution |
| rIA(S) | = | = peak response for insulin aspart from the Standard solution |
| rB28iso(S) | = | = peak response for B28isoAsp insulin aspart from the Standard solution |
| rA21(S) | = | = peak response for A21Asp insulin aspart from the Standard solution |
| rB3(S) | = | = peak response for B3Asp insulin aspart from the Standard solution |
| rB3iso(S) | = | = peak response for B3isoAsp insulin aspart from the Standard solution |
| CS | = | = concentration of the Standard solution |
| CU | = | = concentration of the Sample solution |
Acceptance criteria:
90.0%–104.0% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 6.0% determined on 0.2 g (dried substance)
:
NMT 6.0% determined on 0.2 g (dried substance)
• Related Proteins
Mobile phase, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability:
Proceed as directed in the Assay using the normalization procedure.
Acceptance criteria
Individual impurities:
NMT 1.0% of B28isoAsp insulin aspart; NMT 2.0% total of the peaks due to A21Asp insulin aspart, B3Asp insulin aspart, and B3isoAsp insulin aspart
Total of other impurities:
NMT 1.5%
• Physicochemical Analytical Procedures for Insulins, Limit of High Molecular Weight Proteins  121.1
121.1 :
Meets the requirements of Limit of High Molecular Weight Proteins; NMT 0.5%
:
Meets the requirements of Limit of High Molecular Weight Proteins; NMT 0.5%
SPECIFIC TESTS
• Insulin Assays, Bioidentity Test  121
121 :
Meets the requirements
:
Meets the requirements
• Bacterial Endotoxins Test  85
85 :
It contains NMT 10 USP Endotoxin Units/mg of Insulin Aspart.
:
It contains NMT 10 USP Endotoxin Units/mg of Insulin Aspart.
• Microbial Enumeration Tests  61
61 and Tests for Specified Microorganisms
and Tests for Specified Microorganisms  62
62 :
The total aerobic count does not exceed 300 cfu/g, the test being performed on a portion of about 0.2 g.
:
The total aerobic count does not exceed 300 cfu/g, the test being performed on a portion of about 0.2 g.
• Loss on Drying  731
731
Analysis:
Dry about 200 mg of the sample at 105 for 16 h.
for 16 h.
Acceptance criteria:
NMT 10.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, protected from light, and store in a freezer.
• Labeling:
Label it to indicate that it has been prepared by microbial synthesis.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Edith Chang, Ph.D.
Scientific Liaison (301) 816-8392 |
(BIO12010) Monographs - Biologics and Biotechnology 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison (301) 816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 3871
Pharmacopeial Forum: Volume No. 39(3)