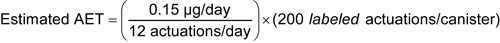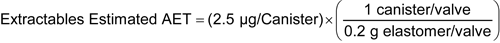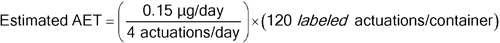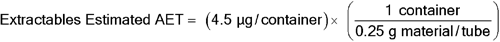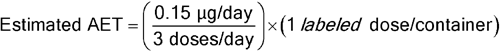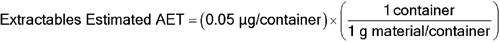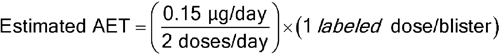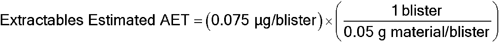Add the following:
INTRODUCTION
This section addresses specific considerations for leachables in orally inhaled and nasal drug products (OINDP), including metered dose inhalers (MDIs); nasal sprays; inhalation solutions, suspensions, and sprays; and dry powder inhalers (DPIs). Although OINDP can be a combination of products that are comprised of drug and device constituent parts, the primary mode of action is typically through the drug. For this reason OINDP are treated as drugs from a regulatory perspective. Regulatory guidance documents and detailed best practice recommendations specific to OINDP are available (1–5). Note that the following discussion is primarily devoted to organic leachables. For consideration of inorganic (i.e., elemental) leachables, see Assessment of Drug Product Leachables Associated with Pharmaceutical Packaging/Delivery Systems  1664
1664 .
.
KEY TERMS
In addition to the key terms listed in  1664
1664 , some additional key terms more specific to OINDP are the following:
, some additional key terms more specific to OINDP are the following:
-
Critical components are packaging components that contact either the drug product formulation or the patient, or that affect the mechanics of the overall performance of the packaging and delivery system, including any necessary secondary packaging. The identification of critical components for a particular OINDP dosage form is the responsibility of the applicant in consultation with appropriate regulatory authorities.
-
Special case compounds are individual (or classes of) compounds that have special safety or historical concerns as drug product leachables in OINDP, and therefore must be evaluated and controlled as leachables (and extractables) by specific analytical techniques and technology-defined thresholds.
Additional terminology and associated definitions specific to OINDP are available in the cited references (1–5).
LEACHABLES ASSESSMENT RATIONALE FOR ORALLY INHALED AND NASAL DRUG PRODUCTS
OINDP are generally categorized as high-risk dosage forms due to safety considerations related to the route of administration and high probability of packaging component interaction with the formulation (see Table 1 in  1664
1664 ). The packaging systems used in these drug products incorporate components of various types, including components composed of polymeric (plastic or elastomeric) raw materials with complex chemical compositions and therefore a variety of potential leachables. Chemical entities may migrate (i.e., leach) into the formulation when there is direct contact with the primary packaging and delivery components for extended periods of time. In certain cases, there is also the potential for leaching from secondary and tertiary packaging. In addition, for OINDP, contact of the delivery device with mucosal tissue (mouth or nasal) is generally expected. Leachables studies for some OINDP may be considered separately for packaging components that are in continuous contact with the formulation (e.g., vials, bottles, blisters, metering valve components) versus those that are only in transient contact (e.g., DPI mouthpiece, MDI mouthpiece).
). The packaging systems used in these drug products incorporate components of various types, including components composed of polymeric (plastic or elastomeric) raw materials with complex chemical compositions and therefore a variety of potential leachables. Chemical entities may migrate (i.e., leach) into the formulation when there is direct contact with the primary packaging and delivery components for extended periods of time. In certain cases, there is also the potential for leaching from secondary and tertiary packaging. In addition, for OINDP, contact of the delivery device with mucosal tissue (mouth or nasal) is generally expected. Leachables studies for some OINDP may be considered separately for packaging components that are in continuous contact with the formulation (e.g., vials, bottles, blisters, metering valve components) versus those that are only in transient contact (e.g., DPI mouthpiece, MDI mouthpiece).
OINDP typically require:
-
A leachables stability study for drug product registration that supports intended storage and use conditions throughout the proposed shelf-life (see Table 1), ideally on primary drug product stability batches manufactured with the same lots of packaging components used in extraction studies (in order to facilitate a leachables–extractables correlation)
-
Sensitive, selective, and fully validated leachables analytical methods
-
Leachables assessments based on safety thresholds [Safety Concern Threshold (SCT): 0.15 µg/day, and Qualification Threshold (QT): 5 µg/day total daily intake (TDI) for an individual organic leachable; however, for exceptions see Special Case Compounds]
-
Complete qualitative and quantitative leachables–extractables correlations (which require that extractables assessments be accomplished on all critical packaging components; see Assessment of Extractables Associated with Pharmaceutical Packaging/Delivery Systems
 1663
1663 )
)
-
Leachables specifications including acceptance criteria (assumes a complete extractables assessment for each critical packaging component). (Note that in many cases routine extractables testing for release of critical components can be used to control drug product leachables in lieu of routine drug product leachables testing, providing that a comprehensive leachables–extractables correlation is established.)
For OINDP dosage forms based on formulations with relatively lower leaching potential for organic compounds (e.g., aqueous formulations, dry powder formulations), the above requirements should be considered and evaluated on a case-by-case basis, including consultations with the appropriate regulatory authorities.
Table 1. Example Stability Storage Conditions and Testing Time Points for an OINDP Registration Leachables Study (5)
| Condition (temperature/relative humidity) |
Time Points (months) |
|---|---|
| 25 ± 2 |
3, 6, 12, 18, 24, 36 (to end of shelf-life) |
| 30 ± 2 |
3, 6, 12, 18, 24, 36 (to end of shelf-life) |
| 40 ± 2 |
3, 6 |
ORALLY INHALED AND NASAL DRUG PRODUCTS DOSAGE FORM TYPES
Metered Dose Inhaler
MDIs or pressurized MDIs (pMDIs) are defined as “drug products that contain active ingredient(s) dissolved or suspended in a propellant, a mixture of propellants, or a mixture of solvent(s), propellant(s), and/or other excipients in compact pressurized aerosol dispensers” (2,5). Typical MDIs include a metal canister (stainless steel or aluminum; coated or uncoated), a fixed-volume metering valve (with plastic or elastomeric components), elastomeric seals, and a plastic actuator or mouthpiece (see Inhalation and Nasal Drug Products: Aerosols, Sprays, and Powders—Performance Quality Tests  601
601 ). MDIs are multidose drug container closure and delivery systems that can contain sufficient formulation for up to several hundred actuations (label claim) per container. Because many of the critical packaging components are in continuous contact with an organic solvent-based formulation, the MDI has the highest risk for formulation-packaging component interaction, and therefore the highest risk for leachables, of all OINDP dosage forms (or any other dosage form). Because of the leaching potential of their organic solvent-based formulations, MDIs would typically be expected to show complete qualitative and quantitative leachables–extractables correlations. Leachables in MDIs should be characterized (i.e., identified and quantitated) at levels above a calculated analytical evaluation threshold (AET). An AET can be calculated for any OINDP dosage form with consideration of the SCT for OINDP (i.e., 0.15 µg/day for an individual organic leachable). An example AET calculation for an MDI follows.
). MDIs are multidose drug container closure and delivery systems that can contain sufficient formulation for up to several hundred actuations (label claim) per container. Because many of the critical packaging components are in continuous contact with an organic solvent-based formulation, the MDI has the highest risk for formulation-packaging component interaction, and therefore the highest risk for leachables, of all OINDP dosage forms (or any other dosage form). Because of the leaching potential of their organic solvent-based formulations, MDIs would typically be expected to show complete qualitative and quantitative leachables–extractables correlations. Leachables in MDIs should be characterized (i.e., identified and quantitated) at levels above a calculated analytical evaluation threshold (AET). An AET can be calculated for any OINDP dosage form with consideration of the SCT for OINDP (i.e., 0.15 µg/day for an individual organic leachable). An example AET calculation for an MDI follows.
Given an MDI drug product with 200 labeled actuations per canister, a maximum recommended patient exposure of 12 actuations per day, and a critical valve component mass per valve of 200 mg, for an individual organic leachable derived from this valve component, the following AET can be estimated:
Leachables Estimated AET = 2.5 µg/canister
To convert to an estimated AET, which would be a useful guide for characterizing potential leachables via extraction studies of this particular valve component (see  1663
1663 ):
):
Extractables Estimated AET = 12.5 µg/g
The AET calculation should not be modified to account for variables, such as manufacturing overfill in the canister to compensate for leak rate or fill variability, unless such modification can be scientifically justified.
Analytical methods for leachables testing of MDI drug products can be based on processes such as “cold filtration” of suspension formulations to remove active ingredient and excipient particles (6) or careful venting of the volatile organic propellant, which retains leachables in a residue within the canister (7). Because sample preparation procedures for MDI formulations can be complex and typically require the volatile propellant to be reduced to dryness at some point, creating the possibility for loss of leachables before sample analysis, it is particularly important to demonstrate adequate recoveries of leachables through the use of spiked MDI samples.
Although it is unlikely to contribute leachables to the emitted drug product aerosol plume, potential patient exposure to chemical entities from the MDI plastic actuator or mouthpiece should be assessed at a threshold of 20 µg/g (see  1663
1663 ). Additional studies and references required to assess patient exposure to actuator- or mouthpiece-derived chemicals include reference to indirect food additive regulations and the application of Biological Reactivity Tests, In Vitro
). Additional studies and references required to assess patient exposure to actuator- or mouthpiece-derived chemicals include reference to indirect food additive regulations and the application of Biological Reactivity Tests, In Vitro  87
87 and Biological Reactivity Tests, In Vivo
and Biological Reactivity Tests, In Vivo  88
88 (2). Note that “spacers” and other devices designed for use with MDIs should also be characterized if a particular device is specified on the drug product label (3).
(2). Note that “spacers” and other devices designed for use with MDIs should also be characterized if a particular device is specified on the drug product label (3).
When constructed from materials acceptable for food contact, MDI actuators and mouthpieces, “spacers”, and other components and devices specified in the drug product labeling generally only require appropriate characterization (i.e., extraction studies and routine extractables testing) in order to assure continued consistent composition of the component or device.
-
Development and validation of surface organic residue release tests for incoming uncoated metal canisters, with appropriate acceptance criteria
-
Development and validation of extractables release tests for the inner surfaces of incoming coated canisters, with appropriate acceptance criteria
-
Development and validation of extractables release tests for incoming metering valve critical components, with appropriate acceptance criteria
-
Development and validation of extractables profile release tests for incoming actuators or mouthpieces, with appropriate qualitative and quantitative acceptance criteria.
Nasal Sprays
Nasal sprays are defined as “drug products that contain active ingredients dissolved or suspended in a formulation, typically aqueous-based, which can contain other excipients and are intended for use by nasal inhalation” (1,5). Nasal sprays include a plastic container and components (usually plastic) that are responsible for formulation metering, atomization, and delivery to the patient (see  601
601 ). Critical components include those that are in constant contact with the formulation (e.g., the container, dip tube) and components that are in the liquid pathway during actuation of the device and that do not permit quick evaporation of residual surface liquid (4). Because nasal sprays are typically aqueous-based formulations, and the vast majority of potential organic leachables are relatively lipophilic, the risk for formulation-packaging component interaction is lower relative to the organic propellant-based MDIs, and the risk for organic leachables is lower. Leachables in nasal sprays should be characterized (i.e., identified and quantitated) at levels above a calculated AET. An AET can be calculated for any OINDP dosage form with consideration of the SCT for OINDP (i.e., 0.15 µg/day for an individual organic leachable). An example AET calculation for a nasal spray follows.
). Critical components include those that are in constant contact with the formulation (e.g., the container, dip tube) and components that are in the liquid pathway during actuation of the device and that do not permit quick evaporation of residual surface liquid (4). Because nasal sprays are typically aqueous-based formulations, and the vast majority of potential organic leachables are relatively lipophilic, the risk for formulation-packaging component interaction is lower relative to the organic propellant-based MDIs, and the risk for organic leachables is lower. Leachables in nasal sprays should be characterized (i.e., identified and quantitated) at levels above a calculated AET. An AET can be calculated for any OINDP dosage form with consideration of the SCT for OINDP (i.e., 0.15 µg/day for an individual organic leachable). An example AET calculation for a nasal spray follows.
Given a nasal spray drug product with 120 labeled actuations per container, a maximum recommended patient exposure of 4 actuations per day, and a critical component (plastic dip tube) mass of 250 mg, for an individual organic leachable derived from this component, the following AET can be estimated:
Leachables Estimated AET = 4.5 µg/container
Given a total fill volume of 10 mL:
Estimated AET = (4.5 µg/container)/(10 mL/container)
Estimated AET = 0.45 µg/mL
To convert to an estimated AET, which would be a useful guide for characterizing potential leachables via extraction studies of this particular plastic dip tube (see  1663
1663 ):
):
Extractables Estimated AET = 18 µg/g
The AET calculation should not be modified to account for variables, such as manufacturing overfill, unless such modification can be scientifically justified.
All nasal spray packaging system critical components should be subjected to extractables assessments (see  1663
1663 ). Potential patient exposure to chemical entities from nasal spray critical components not in continuous contact with the drug product formulation should be assessed at a threshold of 20 µg/g (see
). Potential patient exposure to chemical entities from nasal spray critical components not in continuous contact with the drug product formulation should be assessed at a threshold of 20 µg/g (see  1663
1663 ). Additional studies and references required to assess patient exposure to nonformulation contact critical component derived chemicals include reference to indirect food additive regulations and the application of
). Additional studies and references required to assess patient exposure to nonformulation contact critical component derived chemicals include reference to indirect food additive regulations and the application of  87
87 and
and  88
88 (1).
(1).
When constructed from materials acceptable for food contact, nasal spray critical components not in continuous contact with the drug product formulation generally only need to be appropriately characterized (i.e., extraction studies and routine extractables testing) in order to assure continued consistent composition of the component.
-
Development and validation of extractables release tests for incoming container closure and pump critical components, with appropriate qualitative and quantitative acceptance criteria.
Inhalation Solutions, Suspensions, and Sprays
Inhalation solutions, suspensions, and sprays are defined as “drug products that contain active ingredients dissolved or suspended in a formulation, typically aqueous-based, which can contain other excipients and are intended for use by oral inhalation” (1,5). Inhalation solutions and suspensions are intended for use with a nebulizer (1,5). Inhalation sprays, like MDIs and nasal sprays, are combination products where the components responsible for the metering, atomization, and delivery of the formulation to the patient are a part of the container closure system (1,5). Critical components include components that are in constant contact with the formulation and components that are in the liquid pathway during actuation of the device and that do not permit quick evaporation of residual surface liquid (3). Leachables in inhalation sprays should be characterized (i.e., identified and quantitated) at levels above a calculated AET. An AET can be calculated for any OINDP dosage form with consideration of the SCT for OINDP (i.e., 0.15 µg/day for an individual organic leachable). An example AET calculation for an inhalation spray follows.
Given an inhalation spray drug product with 120 labeled actuations per container, a maximum recommended patient exposure of 4 actuations per day, and a critical component (plastic dip tube) mass of 400 mg, for an individual organic leachable derived from this component, the following AET can be estimated:
Leachables Estimated AET = 4.5 µg/container
Given a total fill volume of 4.5 mL:
Estimated AET = (4.5 µg/container)/(4.5 mL/container)
Estimated AET = 1 µg/mL
To convert to an estimated AET, which would be a useful guide for characterizing potential leachables via extraction studies of this particular plastic dip tube (see  1663
1663 ):
):
Extractables Estimated AET = 11.3 µg/g
The AET calculation should not be modified to account for variables, such as manufacturing overfill, unless such modification can be scientifically justified.
Because inhalation solutions and suspensions are similar to nasal spray and inhalation spray drug products in that they are typically aqueous-based formulations, and the vast majority of potential organic leachables are relatively lipophilic, the risk for formulation-packaging component interaction is lower relative to the organic propellant-based MDIs, and the risk for organic leachables is lower. However, unlike MDIs, nasal and inhalation spray drug products, inhalation solutions, and suspensions are typically packaged in plastic unit dose containers (i.e., nebules). Leaching can potentially occur from the unit dose container [e.g., low-density polyethylene (LDPE)], which is in long-term continuous contact with the drug product formulation. It is also possible that organic chemical entities associated with paper labels, adhesives, inks, etc. in direct contact with the permeable unit dose container can migrate through the container and into the formulation. Leachables from tertiary packaging systems (e.g., cardboard shipping containers) are also possible. Leachables in inhalation solutions and suspensions should be characterized (i.e., identified and quantitated) at levels above a calculated AET. An AET can be calculated for any OINDP dosage form with consideration of the SCT for OINDP (i.e., 0.15 µg/day for an individual organic leachable). An example AET calculation for an inhalation solution follows.
Given an inhalation solution with 3 mL of drug product contained in a LDPE unit dose vial (1 g total weight of LDPE), with a maximum recommended patient exposure of three vials per day, for an individual organic leachable derived from this component, the following AET can be estimated:
Leachables Estimated AET = 0.05 µg/container
Estimated AET = (0.05 µg/container)/(3 mL/container)
Estimated AET = 0.017 µg/mL
To convert to an estimated AET, which would be a useful guide for characterizing potential leachables via extraction studies of this particular plastic unit dose vial (see  1663
1663 ):
):
Extractables Estimated AET = 0.05 µg/g
The challenge of characterizing drug product leachables at levels of 17 ng/mL in an aqueous drug product is considerable, even given the capabilities of modern analytical chemistry. For this particular inhalation solution example, it might be appropriate to implement a simulation study (see  1663
1663 and
and  1664
1664 ) to facilitate the discovery and identification of probable leachables, with actual drug product leachables being quantitated (if required) with high-sensitivity target compound analytical techniques and methods.
) to facilitate the discovery and identification of probable leachables, with actual drug product leachables being quantitated (if required) with high-sensitivity target compound analytical techniques and methods.
All inhalation solution, suspension, and spray packaging system critical components should be subjected to extractables assessments (see  1663
1663 ). Potential patient exposure to chemical entities from inhalation solution, suspension, and spray critical components not in continuous contact with the drug product formulation should be assessed at a threshold of 20 µg/g (see
). Potential patient exposure to chemical entities from inhalation solution, suspension, and spray critical components not in continuous contact with the drug product formulation should be assessed at a threshold of 20 µg/g (see  1663
1663 ). Additional studies and references required to assess patient exposure to nonformulation contact critical component-derived chemicals include reference to indirect food additive regulations and application of
). Additional studies and references required to assess patient exposure to nonformulation contact critical component-derived chemicals include reference to indirect food additive regulations and application of  87
87 and
and  88
88 (1). When constructed from materials acceptable for food contact, inhalation solution, suspension, and spray critical components not in continuous contact with the drug product formulation generally only need be appropriately characterized (i.e., extraction studies and routine extractables testing) in order to assure continued consistent composition of the component. Critical components of nebulizers and other devices designed for use with inhalation solutions and suspensions should also be characterized with respect to extractables and leachables if a particular device is specified in the drug product labeling.
(1). When constructed from materials acceptable for food contact, inhalation solution, suspension, and spray critical components not in continuous contact with the drug product formulation generally only need be appropriately characterized (i.e., extraction studies and routine extractables testing) in order to assure continued consistent composition of the component. Critical components of nebulizers and other devices designed for use with inhalation solutions and suspensions should also be characterized with respect to extractables and leachables if a particular device is specified in the drug product labeling.
-
Development and validation of extractables release tests for incoming container closure and pump critical components, with appropriate qualitative and quantitative acceptance criteria
-
Consideration of validated tests for probable leachables from labels, inks and adhesives, etc., with appropriate acceptance criteria (should these be appropriate and applicable).
Dry Powder Inhalers and Inhalation Powders
DPIs are defined as “drug products designed to dispense powders for inhalation” (2,5). The drug substance in an inhalation powder has a particle size distribution in the respirable range, and may be a physical mixture of active pharmaceutical ingredient(s) with carrier particles or a formulated combination of active ingredient and excipients (see  601
601 ). The powder may be contained in a unit dose packaging system (e.g., capsule, blister), or reside in bulk in a reservoir inside the delivery device itself. In the latter case, the dose is metered by the device. The delivery device may actively disperse the powder from the container or rely on patient inspiration to supply the energy necessary to disperse the particles. The components and the design of the device are integral to the aerosol characteristics (i.e., mass and particle size distribution) of the formulation delivered to the patient. There is a wide diversity of DPI designs and characteristics (2).
). The powder may be contained in a unit dose packaging system (e.g., capsule, blister), or reside in bulk in a reservoir inside the delivery device itself. In the latter case, the dose is metered by the device. The delivery device may actively disperse the powder from the container or rely on patient inspiration to supply the energy necessary to disperse the particles. The components and the design of the device are integral to the aerosol characteristics (i.e., mass and particle size distribution) of the formulation delivered to the patient. There is a wide diversity of DPI designs and characteristics (2).
Of all OINDP, the DPI has the lowest risk of exposing a patient to leachables at significant levels. The reasons for this are:
-
The DPI drug product formulation is a dry powder, and contains no solvent, either organic or aqueous, which can promote leaching of organic (or inorganic) chemical entities.
-
In a unit dose DPI, the drug product formulation is contained in a separate packaging system, and is usually only in transient contact with critical components of the device itself.
The most likely source of leachables in a unit dose DPI would be the material composing the unit dose container, such as a foil laminate blister or capsule material, or the material composing the drug product reservoir in a multidose DPI (including antistatic surface additives). Leaching would have to occur either via direct contact of the drug product powder with the packaging material, via volatilization of organic chemical entities from the container closure material with deposition on the dry powder, or via migration of organic chemical entities through the primary packaging material with deposition on the dry powder. The possibility of observing leachables from the DPI unit dose container is best evaluated with detailed extraction studies on the container material to identify potential leachables, which could possibly migrate to the dry powder by either solid-solid contact or volatilization and have potential safety concerns.
The device and packaging materials are typically evaluated for potential leachables by extraction and simulation studies (see  1663
1663 ) to determine whether there are chemical entities at levels that would pose a safety concern. The evaluation of materials that contain the inhalation powder must consider the inks and any other processing aids used in the manufacture of the container so that all potential leachables are characterized. The types of compounds of greatest concern for inhalation powders are those that may migrate from the primary packaging (i.e., unit dose container or multidose reservoir) into the formulation. Extraction and simulation studies should consider all possible mechanisms of leaching, including volatilization. Actual and potential leachables in inhalation powders derived from critical components of the packaging system or device that may have continuous long-term contact with the drug product formulation should be characterized (i.e., identified and quantitated) at levels above a calculated AET. An AET can be calculated for any OINDP dosage form with consideration of the SCT for OINDP (i.e., 0.15 µg/day for an individual organic leachable). An example AET calculation for an inhalation powder follows.
) to determine whether there are chemical entities at levels that would pose a safety concern. The evaluation of materials that contain the inhalation powder must consider the inks and any other processing aids used in the manufacture of the container so that all potential leachables are characterized. The types of compounds of greatest concern for inhalation powders are those that may migrate from the primary packaging (i.e., unit dose container or multidose reservoir) into the formulation. Extraction and simulation studies should consider all possible mechanisms of leaching, including volatilization. Actual and potential leachables in inhalation powders derived from critical components of the packaging system or device that may have continuous long-term contact with the drug product formulation should be characterized (i.e., identified and quantitated) at levels above a calculated AET. An AET can be calculated for any OINDP dosage form with consideration of the SCT for OINDP (i.e., 0.15 µg/day for an individual organic leachable). An example AET calculation for an inhalation powder follows.
Given a DPI containing 13 mg of inhalation powder in a unit dose blister with 50 mg of blister material either in direct contact with the formulation or capable of volatilizing leachables into the headspace above the formulation, with a maximum recommended daily exposure of 2 actuations per day, for an individual organic leachable derived from this material, the following AET can be estimated:
Leachables Estimated AET = 0.075 µg/blister
To convert relative to the total mass of drug product in a blister:
Estimated AET = (0.075 µg/blister)/(0.013 µg drug product/blister)
Estimated AET = 5.8 µg/g drug product
To convert to an estimated AET, which would be a useful guide for characterizing potential leachables via extraction studies of this particular blister material (see  1663
1663 ):
):
Extractables Estimated AET = 1.5 µg/g
The challenge of characterizing drug product leachables at levels of 5.8 µg/g in an inhalation powder is considerable, even given the capabilities of modern analytical chemistry. For this particular DPI example, it might be appropriate to implement a simulation study (see  1663
1663 and
and  1664
1664 ) to facilitate the discovery and identification of probable leachables from the blister material, with actual drug product leachables being quantitated (if required) with high-sensitivity target compound analytical techniques and methods.
) to facilitate the discovery and identification of probable leachables from the blister material, with actual drug product leachables being quantitated (if required) with high-sensitivity target compound analytical techniques and methods.
All inhalation powder packaging system and DPI device critical components should be subjected to extractables assessments (see  1663
1663 ). Potential patient exposure to chemical entities from inhalation powder packaging system and DPI device critical components not in continuous contact with the drug product formulation should be assessed at a threshold of 20 µg/g (see
). Potential patient exposure to chemical entities from inhalation powder packaging system and DPI device critical components not in continuous contact with the drug product formulation should be assessed at a threshold of 20 µg/g (see  1663
1663 ). Additional studies might be required to assess patient exposure to nonformulation contact critical component derived chemicals, including reference to food additive regulations and application of
). Additional studies might be required to assess patient exposure to nonformulation contact critical component derived chemicals, including reference to food additive regulations and application of  87
87 and
and  88
88 (2).
(2).
When constructed from materials acceptable for food contact, inhalation powder packaging system and DPI device critical components not in continuous contact with the drug product formulation generally only need be appropriately characterized (i.e., extraction studies and routine extractables testing) to assure continued consistent composition of the component.
-
Development and validation of extractables release tests for incoming inhalation powder packaging system and DPI device critical components, with appropriate qualitative and quantitative acceptance criteria.
ADDITIONAL CONSIDERATIONS
Analytical Uncertainty
An AET is that concentration above which unknown leachables should be characterized and reported for toxicological assessment. Target leachables (previously characterized as potential or probable leachables from extractables or simulation studies) will have known safety profiles and previously established leachables thresholds. In addition, reference compounds for previously characterized potential leachables will allow for accurate and precise quantitation of those target leachables as actual drug product leachables. Characterization of unknown leachables requires consideration of analytical uncertainty, as the location of an AET in a given leachables profile (e.g., a gas chromatography/mass spectrometry [GC/MS] chromatogram) must be accomplished relative to an internal standard(s) within the leachables profile. Analytical uncertainty for a particular analytical technique or method can be estimated based on the analysis of a series of reference compounds to create a response factor database. The reference compounds included in this database should represent all known potential leachables (i.e., as determined from extractables assessments). For OINDP, it is recommended (3,5) that the estimated AET be lowered by a factor defined as 1% relative standard deviation in an appropriately constituted response factor database, or a factor of 50% of the estimated AET, whichever is greater. Detailed examples of response factor databases and AET determinations are available (3,5).
Special Case Compounds
Polycyclic Aromatic Hydrocarbons (PAHs) or Polynuclear Aromatics (PNAs), N-nitrosamines, and 2-mercaptobenzothiozole (2-MBT) are considered to be “special case” compounds (i.e., compounds with special safety and historical concerns), requiring special characterization studies using specific analytical techniques and methods (1–3,5). Thresholds for characterization of these compounds as extractables or leachables in OINDP are typically based on the limits of these specific analytical techniques and methods. Table 2 lists the PNAs and N-nitrosamines that, along with 2-MBT, are typically investigated as extractables and leachables in OINDP.
Table 2. PAHs, PNAs, and N-Nitrosamines Typically Investigated as Extractables and Leachables for OINDP
| Target PAHs/PNAs |
Target N-nitrosamines |
|---|---|
| Naphthalene |
N-Nitrosodimethylamine |
| Acenaphthylene |
N-Nitrosodiethylamine |
| Acenaphthene |
N-Nitrosodi-n-butylamine |
| Fluorene |
N-Nitrosomorpholine |
| Phenanthrene |
N-Nitrosopiperidine |
| Anthracene |
N-Nitrosopyrrolidine |
| Fluoranthene |
— |
| Pyrene |
— |
| Benzo(a)anthracene |
— |
| Chrysene |
— |
| Benzo(b)fluoranthene |
— |
| Benzo(k)fluoranthene |
— |
| Benzo(e)pyrene |
— |
| Benzo(a)pyrene |
— |
| Indeno(123-cd)pyrene |
— |
| Dibenzo(ah)anthracene |
— |
| Benzo(ghi)perylene |
— |
PNAs have been associated with carbon black filler used in many types of elastomer. Analysis of PNAs, either as elastomer extractables or as drug product leachables, usually involves quantitative extraction followed by highly specific and sensitive analysis of resulting extracts. GC/MS with selected-ion-monitoring has been reported for analysis of target PNAs as leachables in MDI drug products, for example (6). N-Nitrosamines are reaction products between specific organic precursor molecules, secondary amines (R2NH), and a “nitrosating agent”. In the compounding of rubber, secondary amines are likely formed from certain vulcanization accelerators such as thiurams and dithiocarbamates. Potential nitrosating agents include NO+, N2O3, N2O4, etc., certain of which can be formed from commonly used chemicals such as sodium nitrite (NaNO2), which has many industrial uses. Analysis of N-nitrosamines in rubber as potential leachables involves quantitative extraction followed by analysis of extracts with gas chromatography/thermal energy analysis detection (GC/TEA) (8). Analysis of N-nitrosamines as leachables in MDI drug products using GC/TEA has been reported (7). The 2-MBT is a vulcanization accelerator, which is used in certain sulfur-cured elastomers, and can be analyzed by extraction followed by LC/MS (5).
REFERENCES
-
Guidance for Industry. Nasal Spray and Inhalation Solution, Suspension, and Spray Drug Products—Chemistry, Manufacturing, and Controls Documentation. U.S. Department of Health and Human Services, Food and Drug Administration; Rockville, MD, July 2002.
-
Draft Guidance for Industry. Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) Drug Products—Chemistry, Manufacturing, and Controls Documentation. U.S. Department of Health and Human Services, Food and Drug Administration; Rockville, MD, May 1999.
-
Leachables and Extractables Working Group. Safety thresholds and best practices for extractables and leachables in orally inhaled and nasal drug products. Product Quality Research Institute (PQRI); Arlington, VA, 6 September 2006. http://www.pqri.org/pdfs/LE_Recommendations_to_FDA_09-29-06.pdf. Accessed 19 March 2013.
-
Reviewer Guidance for Nebulizers, Metered Dose Inhalers, Spacers, and Actuators. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Devices and Radiological Health; Rockville, MD, October 1993.
-
Ball D, Norwood D, Stults C, Nagao L, eds. Leachables and Extractables Handbook. New York: J. Wiley and Sons: 2012.
-
Norwood D, Prime D, Downey B, et al. Analysis of polycyclic aromatic hydrocarbons in metered dose inhaler drug formulations by isotope dilution gas chromatography/mass spectrometry. J Pharm Biomed Anal. 1995;13(3): 293.
-
Norwood D, Mullis J, Feinberg T, et al. N-Nitrosamines as “special case” leachables in a metered dose inhaler drug product. PDA J Pharm Sci Technol. 2009;63(4): 307.
-
AOAC International Official Methods of Analysis. N-Nitrosamines in baby bottle rubber nipples. Official Method 987.05, Chapter 48, 7–8; Gaithersburg, MD, 2000.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Desmond G. Hunt, Ph.D.
Senior Scientific Liaison (301) 816-8341 |
(GCPS2010) General Chapters - Packaging Storage and Distribution |
USP38–NF33 Supplement : No. 1 Page 7193
Pharmacopeial Forum: Volume No. 39(5)