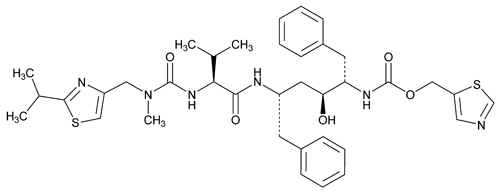
Ritonavir
C37H48N6O5S2 720.94
2,4,7,12-Tetraazatridecan-13-oic acid, 10-hydroxy-2-methyl-5-(1-methylethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-5-thiazolylmethyl ester [5S-(5R*,8R*,10R*,11R*)]-;
5-Thiazolylmethyl [( S)-
S)- -[(1S,3S)-1-hydroxy-3-[(2S)-2-[3-[(2-isopropyl-4-thiazolyl)methyl]-3-methylureido]-3-methylbutyramido]-4-phenylbutyl]phenethyl]carbamate
-[(1S,3S)-1-hydroxy-3-[(2S)-2-[3-[(2-isopropyl-4-thiazolyl)methyl]-3-methylureido]-3-methylbutyramido]-4-phenylbutyl]phenethyl]carbamate


 [155213-67-5].
[155213-67-5].
(ri toe' na vir).
C37H48N6O5S2 720.94
2,4,7,12-Tetraazatridecan-13-oic acid, 10-hydroxy-2-methyl-5-(1-methylethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-5-thiazolylmethyl ester [5S-(5R*,8R*,10R*,11R*)]-;
5-Thiazolylmethyl [(
DEFINITION
Ritonavir contains NLT 97.0% and NMT 102.0% of C37H48N6O5S2, calculated on the anhydrous basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution is within 2% of the retention time of the major peak of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
4.1 mg/mL of monobasic potassium phosphate in water
Solution B:
Acetonitrile, tetrahydrofuran (inhibitor-free), n-butanol, and Solution A (18:8:5:69)
Solution C:
Acetonitrile, tetrahydrofuran (inhibitor-free), n-butanol, and Solution A (47:8:5:40)
Mobile phase:
See the gradient table below.
[Note—Because of the high dependence of retention time and selectivity on the Mobile phase composition, the volumes should be accurately measured. Excessive or continued helium sparging must be avoided. Store the Mobile phase in a tightly sealed container when not in use. ]
| Time (min) |
Solution B (%) |
Solution C (%) |
|---|---|---|
| 0 | 100 | 0 |
| 60 | 100 | 0 |
| 120 | 0 | 100 |
| 120.1 | 100 | 0 |
| 155 | 100 | 0 |
Diluent:
Acetonitrile and Solution A (1:1)
Standard stock solution:
2.0 mg/mL of USP Ritonavir RS in Diluent. [Note—This solution may be kept for 5 days if refrigerated. ]
Standard solution 1:
0.10 mg/mL of USP Ritonavir RS from the Standard stock solution diluted with Diluent
Standard solution 2:
0.025 mg/mL of USP Ritonavir RS from Standard solution 1 diluted with Diluent
Sample solution:
0.025 mg/mL of Ritonavir in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 240 nm
Column:
4.6-mm × 15-cm; 3-µm packing L26
Column temperature:
60
Flow rate:
1 mL/min
Injection size:
50 µL
Run time:
40 min
System suitability
Sample:
Standard solution 2
[Note—The retention time for ritonavir is 30–35 min. ]
Suitability requirements
Capacity factor, k':
NLT 13
Column efficiency:
NLT 5000 theoretical plates
Tailing factor:
0.8–1.2
Relative standard deviation:
2.0%
Analysis
Samples:
Standard solution 2 and Sample solution
Calculate the percentage of C37H48N6O5S2 in the portion of Ritonavir taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Ritonavir RS in Standard solution 2 (mg/mL) |
| CU | = | = concentration of Ritonavir in the Sample solution (mg/mL) |
Acceptance criteria:
97.0%–102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.2%, determined on 1.0 g
:
NMT 0.2%, determined on 1.0 g
• Heavy Metals, Method II  231
231 :
NMT 20 ppm, using 1.0 g of Ritonavir and 2 mL of Standard Lead Solution (10 ppm Pb) in the Standard Preparation
:
NMT 20 ppm, using 1.0 g of Ritonavir and 2 mL of Standard Lead Solution (10 ppm Pb) in the Standard Preparation
Organic Impurities
[Note—Ritonavir is alkali sensitive. All glassware should be prerinsed with distilled water before use to remove residual detergent contamination. ]
• Procedure
Solution A, Solution B, Solution C, Mobile phase, Diluent, Standard stock solution, and Standard solution 1:
Prepare as directed in the Assay.
Identity solution:
1 mg/mL of USP Ritonavir Related Compounds Mixture RS in Diluent
Standard solution 2:
5 µg/mL of USP Ritonavir RS, from Standard solution 1 in Diluent. [Note—Stable for 48 h. ]
Sample solution:
1 mg/mL of Ritonavir in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 240 nm
Column:
4.6-mm × 15-cm; 3-µm packing L26
Column temperature:
60
Flow rate:
1 mL/min
Injection size:
50 µL
Run time
Standard solution 2:
40 min
System suitability
Samples:
Identity solution and Standard solution 2
[Note—The retention time of ritonavir is 30–35 min. See Impurity Table 1 for relative retention times. ]
Suitability requirements
Resolution:
NLT 1.0 between impurity E and impurity F, Identity solution
Peak-to-valley ratio:
NLT 1 for ritonavir and impurity N (regioisomer), Identity solution
Capacity factor, k':
NLT 13, Standard solution 2
Column efficiency:
NLT 5000 theoretical plates, Standard solution 2
Tailing factor:
0.8–1.2, Standard solution 2
Relative standard deviation
NMT 3.0%, Standard solution 2
Analysis
Samples:
Diluent, Identity solution, Standard solution 2, and Sample solution
Calculate the percentage of each impurity in the portion of Ritonavir taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from Standard solution 2 |
| CS | = | = concentration of Standard solution 2 (mg/mL) |
| CU | = | = nominal concentration of Ritonavir in the Sample solution (mg/mL) |
| F | = | = relative response factor |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 1.0%
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Mixture of 2,4-Wing acid and monoacyl valine (A + B) | 0.07 | 1.0 | 0.1 |
| Monoacylacetamide (C) | 0.15 | 1.0 | 0.1 |
| 5-Wing diacyl (D) | 0.24 | 1.37 | 0.1 |
| Oxidation impurity (E) | 0.36 | 1.0 | 0.3 |
| Acid hydrolysis product (F) | 0.39 | 0.73 | 0.1 |
| Ritonavir hydroperoxide (G) | 0.45 | 1.0 | 0.1 |
| Acid/base by-product (H) | 0.47 | 0.76 | 0.1 |
| Ethyl analog (I) | 0.64 | 1.0 | 0.1 |
| Mixture of Boc-monoacyl and monoacyl isobutyl carbamate (J + K) | 0.81 | 0.74 | 0.1 |
| Base cyclization product (L) | 0.87 | 0.53 | 0.1 |
| 2,4-Wing isobutyl ester (M) | 0.94 | 1.0 | 0.1 |
| Regioisomer (N) | 1.05 | 1.0 | 0.1 |
| Isomer #2 (O) | 1.11 | 1.0 | 0.3 |
| Di-monoacyl urea (P) | 1.14 | 1.0 | 0.1 |
| Isomer #4 (Q) | 1.23 | 1.0 | 0.1 |
| Isomer #1 (R) | 1.32 | 1.0 | 0.1 |
| Di-monoacyl valine urea (S) | 1.62 | 1.0 | 0.1 |
| 2,4-Wing diacyl (T) | 2.87 | 0.73 | 0.2 |
| Triacyl impurity (U) | 3.20 | 1.0 | 0.1 |
| Any other individual impurity | — | 1.0 | 0.1 |
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
NMT 0.5%, determined on 0.500 g
:
NMT 0.5%, determined on 0.500 g
• X-Ray Diffraction  941
941 :
The X-ray diffraction pattern conforms to that of USP Ritonavir RS, if used in a solid dosage form.
:
The X-ray diffraction pattern conforms to that of USP Ritonavir RS, if used in a solid dosage form.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers. Store between 5 and 30
and 30 .
.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Leonel M. Santos, Ph.D.
Senior Scientific Liaison 1-301-816-8168 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4579
Pharmacopeial Forum: Volume No. 37(4)