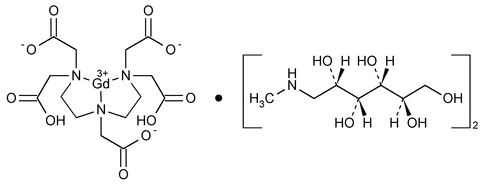
Gadopentetate Dimeglumine Injection
» Gadopentetate Dimeglumine Injection is a sterile solution of gadopentetate dimeglumine in Water for Injection. It contains not less than 90.0 percent and not more than 110.0 percent of the labeled amount of gadopentetate dimeglumine (C14H20GdN3O10·2C7H17NO5). It may contain small amounts of Meglumine and Pentetic Acid as stabilizers, and it may contain suitable buffers. Gadopentetate Dimeglumine Injection intended for intravascular use contains no antimicrobial agents.
Packaging and storage—
Preserve in single-dose containers, preferably of Type I glass, protected from light. Store at controlled room temperature.
Labeling—
Label containers of Injection intended for intravascular injection to direct the user to discard any unused portion remaining in the container.
Identification—
A:
Ultraviolet Absorption  197U
197U —
—
Solutions:
74 mg of USP Gadopentetate Monomeglumine RS per mL, and 94 mg of Gadopentetate Dimeglumine per mL.
Medium:
water.
B:
Dilute a volume of Injection with water to obtain a test solution having a concentration of 35 mg per mL. Separately apply 10 µL of this solution and 10 µL of a Standard solution containing 28 mg of USP Gadopentetate Monomeglumine RS per mL in a meglumine solution (0.075 in 1000) to a thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture. Allow the spots to dry, and develop the chromatogram in a solvent system consisting of a mixture of 1,4-dioxane, water, and ammonium hydroxide (70:30:2) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate. Dip the plate in a detection reagent prepared by mixing 100 mg of ninhydrin and 250 mg of cadmium acetate with 1.0 mL of glacial acetic acid and diluting with alcohol to 50 mL. Heat the plate at 120
) coated with a 0.25-mm layer of chromatographic silica gel mixture. Allow the spots to dry, and develop the chromatogram in a solvent system consisting of a mixture of 1,4-dioxane, water, and ammonium hydroxide (70:30:2) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate. Dip the plate in a detection reagent prepared by mixing 100 mg of ninhydrin and 250 mg of cadmium acetate with 1.0 mL of glacial acetic acid and diluting with alcohol to 50 mL. Heat the plate at 120 for 10 minutes, and locate the spots by examining the plate in daylight: the principal spot obtained from the test solution corresponds in appearance and RF value to the principal spot obtained from the Standard solution (presence of meglumine).
for 10 minutes, and locate the spots by examining the plate in daylight: the principal spot obtained from the test solution corresponds in appearance and RF value to the principal spot obtained from the Standard solution (presence of meglumine).
C:
Prepare the test solution and Standard solution as directed for Identification test A. Transfer 500 mL of 1 N sulfuric acid at 0 to a 1000-mL conical flask, immerse in an ice bath, and add 50 g of ceric sulfate tetrahydrate. Mix until dissolved, filter, and refrigerate (Stock solution A). Transfer 325 mL of 2 N sulfuric acid at 0
to a 1000-mL conical flask, immerse in an ice bath, and add 50 g of ceric sulfate tetrahydrate. Mix until dissolved, filter, and refrigerate (Stock solution A). Transfer 325 mL of 2 N sulfuric acid at 0 to a 500-mL volumetric flask. To 25 g of sodium arsenite, add 150 mL of 1 N sodium hydroxide, mix, and add in small portions to the sulfuric acid. Dilute with water to volume, and refrigerate (Stock solution B). Just prior to use, prepare a spray reagent by mixing equal volumes of Stock solution A and Stock solution B at a temperature not lower than 10
to a 500-mL volumetric flask. To 25 g of sodium arsenite, add 150 mL of 1 N sodium hydroxide, mix, and add in small portions to the sulfuric acid. Dilute with water to volume, and refrigerate (Stock solution B). Just prior to use, prepare a spray reagent by mixing equal volumes of Stock solution A and Stock solution B at a temperature not lower than 10 . [note—Use the spray reagent within five minutes. ] Proceed as directed under Thin-layer Chromatographic Identification Test
. [note—Use the spray reagent within five minutes. ] Proceed as directed under Thin-layer Chromatographic Identification Test  201
201 , except to spray the plate first with the spray reagent and then with a 1% solution of 1,2-phenylenediamine in acetone. Locate the spots by examining the plate in daylight: the principal spot obtained from the test solution corresponds in appearance and RF value to the principal spot obtained from the Standard solution (presence of gadopentetate).
, except to spray the plate first with the spray reagent and then with a 1% solution of 1,2-phenylenediamine in acetone. Locate the spots by examining the plate in daylight: the principal spot obtained from the test solution corresponds in appearance and RF value to the principal spot obtained from the Standard solution (presence of gadopentetate).
Bacterial endotoxins  85
85 —
It contains not more than 25 USP Endotoxin Units per mL of Injection.
—
It contains not more than 25 USP Endotoxin Units per mL of Injection.
pH  791
791 :
between 6.5 and 8.0.
:
between 6.5 and 8.0.
Heavy metals  231
231 —
—
Standard preparation—
Proceed as directed in the test for Heavy metals under Diatrizoate Meglumine.
Test preparation—
In a 50-mL color-comparison tube, mix a volume of Injection, equivalent to 1.0 g of gadopentetate dimeglumine, with 5 mL of 1 N sodium hydroxide, dilute with water to 40 mL, and mix.
Procedure—
Proceed as directed for Procedure in the test for Heavy metals under Diatrizoate Meglumine: the limit is 0.002%.
Meglumine content—
Proceed as directed in the test for Meglumine content under Diatrizoate Meglumine Injection. The meglumine content is between 37.4% and 45.8% of the labeled amount of gadopentetate dimeglumine.
Content of gadolinium—
Cesium chloride solution—
Dissolve 10.0 g of cesium chloride in 100.0 mL of water, and mix.
Blank solution—
Transfer 10.0 mL of Cesium chloride solution and 1.0 mL of hydrochloric acid (spectrophotometric grade) to a 100-mL volumetric flask, dilute with water to volume, and mix.
Standard solutions—
Transfer about 1.153 g of gadolinium (III) oxide, accurately weighed, to a 100-mL volumetric flask, add 2.0 mL of hydrochloric acid to dissolve, dilute with water to volume, and mix. Transfer 3.0, 4.0, and 5.0 mL of this stock solution to separate 50-mL volumetric flasks, and to each flask add 5.0 mL of Cesium chloride solution and 0.5 mL of hydrochloric acid (spectrophotometric grade). Dilute the contents of each flask with water to volume, and mix. These Standard solutions contain, respectively, 600, 800, and 1000 µg of gadolinium per mL.
Test solution—
Treat an accurately measured volume of Injection, equivalent to about 469 mg of gadopentetate dimeglumine, with 0.2 mL of nitric acid in a porcelain crucible, concentrate on a hot plate, char with a burner, and ignite in a muffle furnace at 800 until all black particles disappear (approximately 1 hour). Allow the residue to cool on a refractory surface for about 5 minutes, then equilibrate to room temperature in a desiccator. Dissolve the white residue so obtained in a mixture of 1.0 mL of water and 1.0 mL of hydrochloric acid (spectrophotometric grade) with heating. Transfer this solution to a 100-mL volumetric flask, add 10.0 mL of Cesium chloride solution, dilute with water to volume, and mix.
until all black particles disappear (approximately 1 hour). Allow the residue to cool on a refractory surface for about 5 minutes, then equilibrate to room temperature in a desiccator. Dissolve the white residue so obtained in a mixture of 1.0 mL of water and 1.0 mL of hydrochloric acid (spectrophotometric grade) with heating. Transfer this solution to a 100-mL volumetric flask, add 10.0 mL of Cesium chloride solution, dilute with water to volume, and mix.
Procedure—
Concomitantly determine the absorbances of the Standard solutions and the Test solution at the gadolinium emission line at 368.4 nm, with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-scattering  851
851 ) equipped with a gadolinium hollow-cathode lamp and a nitrous oxide–acetylene flame, using the Blank solution as the blank. Plot the absorbances of the Standard solutions versus their concentrations, in µg per mL, of gadolinium, and draw the straight line best fitting the three plotted points. From the graph so obtained and the absorbance of the Test solution, determine the concentration, in µg per mL, of gadolinium in the Test solution. Calculate the quantity, in µg, of gadolinium in each mL of the Injection taken by the formula:
) equipped with a gadolinium hollow-cathode lamp and a nitrous oxide–acetylene flame, using the Blank solution as the blank. Plot the absorbances of the Standard solutions versus their concentrations, in µg per mL, of gadolinium, and draw the straight line best fitting the three plotted points. From the graph so obtained and the absorbance of the Test solution, determine the concentration, in µg per mL, of gadolinium in the Test solution. Calculate the quantity, in µg, of gadolinium in each mL of the Injection taken by the formula:
100C / V
in which C is the concentration, in µg per mL, of gadolinium in the Test solution; and V is the volume, in mL, of Injection taken. The gadolinium content is between 15.1% and 18.4% of the labeled amount of gadopentetate dimeglumine.
Content of pentetic acid—
Stock solution A—
Transfer about 50 g of sodium acetate and 10 mL of glacial acetic acid to a 1000-mL volumetric flask, and dilute with degassed water to volume. Adjust with 0.1 N sodium hydroxide or glacial acetic acid to a pH of 5.
Stock solution B—
Transfer about 50.8 mg of xylenol orange to a 100-mL volumetric flask, and add degassed water to volume.
Diluting solution—
Transfer 30 mL of Stock Solution A and 3 mL of Stock Solution B to a 200-mL volumetric flask, and dilute with degassed water to volume.
Procedure—
Transfer an accurately measured volume of Injection, equivalent to about 938 mg of gadopentetate dimeglumine, to a suitable container, add 20 mL of water and 10 mL of Diluting solution, and mix. Adjust with 0.1 N sodium hydroxide or glacial acetic acid to a pH of 5. Titrate with 0.001 M gadolinium sulfate solution until the color changes from yellow to reddish violet. Each mL of 0.001 M gadolinium sulfate consumed is equivalent to 0.7867 mg of pentetic acid (C14H23N3O10). The pentetic acid content is between 0.027% and 0.04%.
Other requirements—
It meets the requirements under Injections  1
1 .
.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture containing about 1.37 g of tetrabutylammonium perchlorate in a mixture of acetonitrile and water (120:880). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard preparation—
Transfer about 46.43 mg of USP Gadopentetate monomeglumine RS, accurately weighed, to a 25-mL volumetric flask containing 12.5 mL of 0.1% meglumine solution. Dilute with water to volume, and mix.
Assay preparation—
Transfer an accurately measured volume of Injection, equivalent to about 469 mg of gadopentetate dimeglumine, to a 200-mL volumetric flask. Dilute with water to volume, and mix.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 195-nm detector and a 4.6-mm × 12.5-cm column that contains 5-µm packing L7. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the column efficiency is not less than 800 theoretical plates, the tailing factor is not more than 3.5, and the relative standard deviation for replicate injections is not more than 2.0%.
)—The liquid chromatograph is equipped with a 195-nm detector and a 4.6-mm × 12.5-cm column that contains 5-µm packing L7. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the column efficiency is not less than 800 theoretical plates, the tailing factor is not more than 3.5, and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C14H20GdN3O10·2C7H17NO5 in the portion of Injection taken by the formula:
(938.02 / 742.80)(200C)(RU / RS)
in which 938.02 and 742.80 are the molecular weights of gadopentetate dimeglumine and gadopentetate monomeglumine, respectively; C is the concentration, in mg per mL, of USP Gadopentetate Monomeglumine RS in the Standard preparation; and RU and RS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison 1-301-816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology |
USP35–NF30 Page 3303