Glucosamine, Chondroitin Sulfate Sodium, and Methylsulfonylmethane Tablets
DEFINITION
Glucosamine, Chondroitin Sulfate Sodium, and Methylsulfonylmethane Tablets are prepared from either Glucosamine Hydrochloride, Glucosamine Sulfate Sodium Chloride, Glucosamine Sulfate Potassium Chloride, or a mixture of any of them, with Chondroitin Sulfate Sodium and Methylsulfonylmethane. Tablets contain NLT 90.0% and NMT 120.0% of the labeled amounts of chondroitin sulfate sodium and glucosamine (C6H13NO5) and NLT 90.0% and NMT 110.0% of the labeled amount of methylsulfonylmethane (C2H6O2S).
[Note—Chondroitin Sulfate Sodium is extremely hygroscopic once dried. Avoid exposure to atmosphere, and weigh promptly. ]
IDENTIFICATION
• A. Presence of Glucosamine:
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the test for Content of Glucosamine.
• B. Presence of Chondroitin Sulfate
(See Electrophoresis  726
726 .)
.)
Barium acetate buffer:
Dissolve 25.24 g of barium acetate in 900 mL of water. Adjust with acetic acid to a pH of 5.0, and dilute with water to 1000 mL.
Staining reagent:
0.1% (w/v) toluidine blue in 0.1 M acetic acid
Standard solution:
Use the Standard solution of middle concentration from Content of Chondroitin Sulfate Sodium.
Sample solution:
Prepare as directed in Content of Chondroitin Sulfate Sodium.
Analysis:
Fill the chambers of an electrophoresis apparatus suitable for separations on cellulose acetate membranes1 (a small submarine gel chamber or one dedicated to membrane media) with Barium acetate buffer. Soak a cellulose acetate membrane 5–6 cm × 12–14 cm in Barium acetate buffer for 10 min, or until evenly wetted, then blot dry between two sheets of absorbent paper. Using an applicator2 suitable for electrophoresis, apply equal volumes (0.5 µL) of the Sample solution and Standard solution to the brighter side of the membrane held in position in an appropriate applicator stand or on a separating bridge in the chamber. Ensure that both ends of the membrane are dipped at least 0.5–1.0 cm deep into the buffer chambers. Apply a constant 60 volts (6 mA at the start) for 2 h. [Note—Perform the application of solutions and voltage within 5 min because further drying of the blotted paper reduces sensitivity. ]
Place the membrane in a plastic staining tray, and with the application side down, float or gently immerse in Staining reagent for 5 min. Then stir the solution gently for 1 min. Remove the membrane, and destain in 5% acetic acid until the background clears.
Acceptance criteria:
The principal band from the Sample solution has the same migration as the principal band from the Standard solution. [Note—Document the results by taking a picture within 15 min of completion of destaining. ]
• C. Presence of Methylsulfonylmethane:
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Content of Methylsulfonylmethane.
STRENGTH
• Content of Glucosamine
Diluent:
Transfer 29 µL of acetic acid and 5 mL of acetonitrile to a 100-mL volumetric flask containing 50 mL of water, and dilute with water to volume.
Borate buffer:
0.2 M (76.3 g/L of sodium borate in water) adjusted with hydrochloric acid TS to a pH of 9.5
Acetate buffer:
6.80 g/L of sodium acetate trihydrate in water adjusted with dilute acetic acid to a pH of 5.9. [Note—Buffer must be stored at room temperature and can be warmed to dissolve if crystallization occurs. ]
Derivatizing reagent:
In a 14-mL polypropylene culture tube, dissolve 50 mg of o-phthalaldehyde in 1.25 mL of anhydrous methanol. Add 50 µL of 3-mercaptopropionic acid and 11.2 mL of Borate buffer, and mix gently. Allow to stand in the dark for 30 min before use. [Note—Reagent strength is maintained by adding 10 µL of 3-mercaptopropionic acid every 2 days. Storage should be in the dark, at room temperature, and can be used for NMT 2 weeks. ]
Mobile phase:
Methanol and Acetate buffer (1:9)
Standard solution:
1.0 mg/mL of USP Glucosamine Hydrochloride RS in water. Allow to stand at room temperature for 1 h.
Sample solution:
Transfer an equivalent to 25 mg of glucosamine from NLT 20 Tablets, finely powdered, to a 25-mL volumetric flask, and dilute with Diluent to volume. Mix on a vortex mixer to suspend the powder in solution. Sonicate in a 65 water bath for 20 min. Remove from the bath, stir for 5 min with the aid of a magnetic stirrer, and centrifuge.
water bath for 20 min. Remove from the bath, stir for 5 min with the aid of a magnetic stirrer, and centrifuge.
Chromatographic system
Mode:
LC
Detector:
UV 340 nm
Column:
3.0-mm × 5-cm; packing L1
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Samples:
Five individual aliquots of the Standard solution derivatized as directed in Analysis. Each derivatized aliquot is injected only once.
[Note—The relative retention times for the 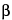 -anomer and the
-anomer and the  -anomer are 1.0 and 1.8, respectively. The retention time for the
-anomer are 1.0 and 1.8, respectively. The retention time for the 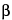 -anomer is NLT 4 min. ]
-anomer is NLT 4 min. ]
Suitability requirements
Relative standard deviation:
NMT 2.0% for five replicate injections
Analysis
Samples:
Standard solution and Sample solution
Transfer 100 µL of the Derivatizing reagent and 100 µL of the Standard solution or the Sample solution to a vial containing 400 µL of Borate buffer, and allow the derivatization to proceed for 1 min. Inject the derivatized solutions immediately after the derivatization reaction.
Calculate the percentage of the labeled amount of glucosamine (C6H13NO5) in the portion of Tablets taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response of the |
| rS | = | = peak response of the |
| CS | = | = concentration of USP Glucosamine Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of glucosamine in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of glucosamine, 179.17 |
| Mr2 | = | = molecular weight of glucosamine hydrochloride, 215.63 |
Acceptance criteria:
90.0%–120.0% of the label claim
• Content of Chondroitin Sulfate Sodium
Standard solutions:
1.5, 1.0, and 0.5 mg/mL of USP Chondroitin Sulfate Sodium RS in water
Sample solution:
Transfer an equivalent to 100 mg of chondroitin sulfate sodium from NLT 20 Tablets, finely powdered, to 60 mL of water, and shake to suspend the powder in solution. Sonicate in a 65 water bath for 20 min. Remove from the bath, stir or shake for 5 min, dilute with water to 100 mL, and centrifuge or pass through a suitable filter.
water bath for 20 min. Remove from the bath, stir or shake for 5 min, dilute with water to 100 mL, and centrifuge or pass through a suitable filter.
Diluent:
Weigh about 297 mg of monobasic potassium phosphate, 492 mg of dibasic potassium phosphate, and 250 mg of polysorbate 80, and transfer into a 1-L beaker. Dissolve in approximately 900 mL of water, and adjust with potassium hydroxide or phosphoric acid to a pH of 7.0 ± 0.2. Dilute with water to 1 L, and mix thoroughly.
Titrimetric system
(See Titrimetry  541
541 .)
.)
Mode:
Photometric titration
Titrant:
1 mg/mL of cetylpyridinium chloride in water
Endpoint detection:
Turbidimetric with photoelectric probe
Analysis:
Transfer 5.0 mL of each Standard solution and the Sample solution to separate titration vessels, and add 25 mL of Diluent to each. Stir until a steady reading is obtained with a photoelectric probe either at 420, 550, or 660 nm. Set the instrument to zero in absorbance mode. Titrate with Titrant using the photoelectric probe to determine the endpoint turbidimetrically. From a linear regression equation, calculated using the volumes of Titrant consumed versus concentrations of the Standard solutions, determine the concentration of chondroitin sulfate sodium in the Sample solution.
Calculate the percentage of chondroitin sulfate sodium in the portion of Tablets taken:
Result = (C/CU) × 100
| C | = | = determined concentration of chondroitin sulfate sodium in the Sample solution (mg/mL) |
| CU | = | = nominal concentration of chondroitin sulfate sodium in the Sample solution (mg/mL) |
Acceptance criteria:
90.0%–120.0% of the label claim
• Content of Methylsulfonylmethane
Diluent:
Transfer 950 mL of methanol to a 1-L volumetric flask. Add 0.60 mL of diethylene glycol methyl ether, and dilute with methanol to volume.
Standard solution:
0.4 mg/mL of USP Methylsulfonylmethane RS in Diluent. Sonicate at 50 for 1 min, and allow to cool to room temperature.
for 1 min, and allow to cool to room temperature.
Sample solution:
Finely powder NLT 20 Tablets. Dissolve a portion of the finely powdered material, equivalent to 1 Tablet, in Diluent, and sonicate for 15 min at 50 . Allow to cool to room temperature. Quantitatively dilute with Diluent to obtain a final concentration of 0.4 mg/mL of methylsulfonylmethane. Transfer 1 mL of the suspension to a 1.5-mL microcentrifuge tube, and centrifuge for 20 s. Use the supernatant.
. Allow to cool to room temperature. Quantitatively dilute with Diluent to obtain a final concentration of 0.4 mg/mL of methylsulfonylmethane. Transfer 1 mL of the suspension to a 1.5-mL microcentrifuge tube, and centrifuge for 20 s. Use the supernatant.
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.53-mm × 30-m capillary column coated with a 5-µm phase G2
Temperature
Column:
120
Injector:
250
Detector:
250
Carrier gas:
Helium
Flow rate:
5 mL/min
Injection size:
1 µL
Injection type
Split ratio, 2:1
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 2.0% for the peak response ratio of methylsulfonylmethane to diethylene glycol methyl ether from replicate injections
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of the labeled amount of methylsulfonylmethane (C2H6O2S) in the portion of Tablets taken:
Result = (RU/RS) × (CS/CU) × 100
| RU | = | = peak area ratio of methylsulfonylmethane to diethylene glycol methyl ether from the Sample solution |
| RS | = | = peak area ratio of methylsulfonylmethane to diethylene glycol methyl ether from the Standard solution |
| CS | = | = concentration of USP Methylsulfonylmethane RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of methylsulfonylmethane in the Sample solution (mg/mL) |
Acceptance criteria:
90.0%–110.0% of of the label claim
PERFORMANCE TESTS
• Disintegration and Dissolution  2040
2040 :
Meet the requirements for Dissolution
:
Meet the requirements for Dissolution
Medium:
Water; 900 mL
Apparatus 2:
75 rpm
Time:
60 min
Determine the percentage of glucosamine dissolved as follows.
Borate buffer, Acetate buffer, Derivatizing reagent, Mobile phase, Chromatographic system, and Analysis:
Proceed as directed in the test for Content of Glucosamine.
Standard solution:
Prepare as directed in the test for Content of Glucosamine. Dilute with a suitable quantity of water, if necessary.
Sample solution:
Use the solution under test.
Calculate the percentage of the labeled amount of glucosamine (C6H13NO5) dissolved:
Result = (rU/rS) × (CS × V/L) × (Mr1/Mr2) × 100
| rU | = | = peak area obtained from the derivatized Sample solution |
| rS | = | = peak area obtained from the derivatized Standard solution |
| CS | = | = concentration of USP Glucosamine Hydrochloride RS in the Standard solution (mg/mL) |
| V | = | = volume of Medium, 900 mL |
| L | = | = label claim of glucosamine (mg/Tablet) |
| Mr1 | = | = molecular weight of glucosamine, 179.17 |
| Mr2 | = | = molecular weight of glucosamine hydrochloride, 215.63 |
Tolerances:
NLT 75% of the labeled amount of glucosamine (C6H13NO5) is dissolved.
Determine the percentage of chondroitin sulfate sodium dissolved as follows.
Titrant, Diluent, Standard solutions, and Analysis:
Proceed as directed in the test for Content of Chondroitin Sulfate Sodium.
Sample solution:
Use the solution under test.
Calculate the percentage of the labeled amount of chondroitin sulfate sodium dissolved:
Result = (C × V/L) × 100
| C | = | = determined concentration of chondroitin sulfate sodium in the Sample solution (mg/mL) |
| V | = | = volume of Medium, 900 mL |
| L | = | = label claim of chondroitin sulfate sodium (mg/Tablet) |
Tolerances:
NLT 75% of the labeled amount of chondroitin sulfate sodium is dissolved.
• Weight Variation  2091
2091 :
Meet the requirements
:
Meet the requirements
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers.
• Labeling:
The label indicates the types of glucosamine salts contained in the article and the species source from which chondroitin was derived. Label it to state the source(s) of chondroitin sulfate sodium, whether bovine, porcine, avian, or a mixture of any of them. The label states on the front panel the content of chondroitin sulfate sodium on the dried basis.
• USP Reference Standards  11
11
USP Methylsulfonylmethane RS
Dimethyl sulfone.
C2H6O2S 94.13
Dimethyl sulfone.
C2H6O2S 94.13
1
Suitable cellulose acetate membranes for electrophoresis are available from Malta Chemetron SRL, Milano, Italy (www.maltachemetron.com); Fluka Chemical Corp., Milwaukee, WI; and DiaSys Corp., Waterbury, CT (www.diasys.com).
2
Suitable applicators are available from DiaSys Corp., Waterbury, CT (www.diasys.com) and Helena Laboratories, Beaumont, TX (www.helena.com).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Natalia Davydova
Scientific Liaison 1-301-816-8328 |
(DS2010) Monographs - Dietary Supplements |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1339
Pharmacopeial Forum: Volume No. 32(4) Page 1138
