INTRODUCTION
This general chapter is provided to determine compliance with the disintegration and dissolution standards for dietary supplements where stated in the individual monographs.
For the purposes of this chapter, dietary supplement dosage forms have been divided into three categories: Vitamin–Mineral Dosage Forms, Botanical Dosage Forms, and Dietary Supplements Other Than Vitamin–Mineral and Botanical Dosage Forms. Vitamin–Mineral Dosage Forms includes articles prepared with vitamins, minerals, or combinations of these dietary ingredients (e.g., USP dietary supplements Class I to Class VI, described below). Botanical Dosage Forms comprises formulations containing ingredients of botanical origin, including plant materials and extracts. Dietary Supplements Other Than Vitamin–Mineral and Botanical Dosage Forms encompasses dietary supplements formulated with lawfully recognized dietary ingredients that are different from those pertaining to the two foregoing categories (e.g., amino acids, chondroitin, and glucosamine.)
Where a dietary supplement represents a combination of the categories mentioned above, and there is a difference between the requirements for the individual categories, the more stringent requirement applies.
Dissolution testing as described in this chapter is a quality-control tool to enable the performance of dietary supplements to be routinely assessed.
DISINTEGRATION
This test is provided to determine whether dietary supplement tablets or capsules disintegrate within the prescribed time when placed in a liquid medium at the experimental conditions presented below. Compliance with the limits on Disintegration stated in the individual monographs for dietary supplements is required except where the label states that the products are intended for use as troches, are to be chewed, or are designed as extended-release dosage forms. Dietary supplements claiming to be extended-release dosage forms must comply with standards other than disintegration to verify that the release of the dietary ingredients from the dosage form is for a defined period of time. Dietary supplements claiming to be extended-release dosage forms shall not be labeled as in compliance with USP unless a USP monograph exists for such product. Determine the type of units under test from the labeling and from observation, and apply the appropriate procedure to 6 or more units.
For purposes of this test, disintegration does not imply complete solution of the unit or even of its active constituent. Complete disintegration is defined as that state in which any residue of the unit, except fragments of insoluble coating or capsule shell, remaining on the screen of the test apparatus or adhering to the lower surface of the disk, if used, is a soft mass having no palpably firm core.
Apparatus
Apparatus A—
Use the Apparatus described under Disintegration  701
701 for tablets or capsules that are not greater than 18 mm long. For larger tablets or capsules, use Apparatus B.
for tablets or capsules that are not greater than 18 mm long. For larger tablets or capsules, use Apparatus B.
Apparatus B—
The apparatus1 consists of a basket-rack assembly, a 1000-mL low-form beaker for the immersion fluid, a thermostatic arrangement for heating the fluid between 35 and 39
and 39 , and a device for raising and lowering the basket in the immersion fluid at a constant frequency rate between 29 and 32 cycles per minute through a distance of not less than 53 mm and not more than 57 mm. The volume of the fluid in the vessel is such that at the highest point of the upward stroke the wire mesh remains at least 15 mm below the surface of the fluid and descends to not less than 25 mm from the bottom of the vessel on the downward stroke. At no time should the top of the basket-rack assembly become submerged. The time required for the upward stroke is equal to the time required for the downward stroke, and the change in stroke direction is a smooth transition rather than an abrupt reversal of motion. The basket-rack assembly moves vertically along its axis. There is no appreciable horizontal motion or movement of the axis from the vertical.
, and a device for raising and lowering the basket in the immersion fluid at a constant frequency rate between 29 and 32 cycles per minute through a distance of not less than 53 mm and not more than 57 mm. The volume of the fluid in the vessel is such that at the highest point of the upward stroke the wire mesh remains at least 15 mm below the surface of the fluid and descends to not less than 25 mm from the bottom of the vessel on the downward stroke. At no time should the top of the basket-rack assembly become submerged. The time required for the upward stroke is equal to the time required for the downward stroke, and the change in stroke direction is a smooth transition rather than an abrupt reversal of motion. The basket-rack assembly moves vertically along its axis. There is no appreciable horizontal motion or movement of the axis from the vertical.
Basket-Rack Assembly—
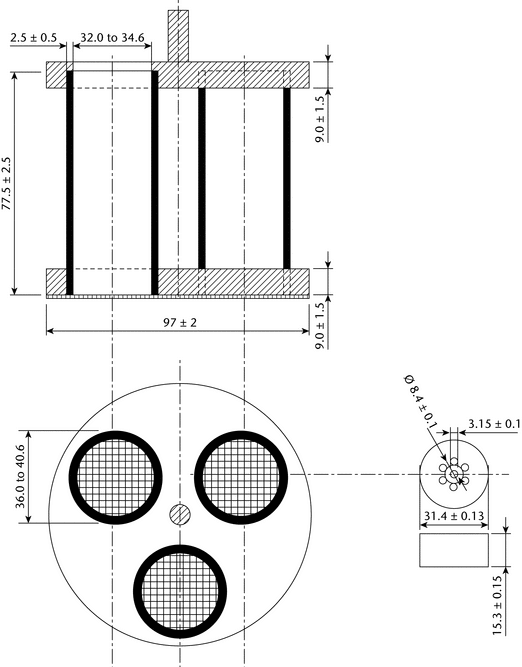
The basket-rack assembly (see Figure 1) consists of three open-ended transparent tubes, each 77.5 ± 2.5 mm long and having an inside diameter of 32.0 to 34.6 mm and a wall 2.0 to 3.0 mm thick; the tubes are held in a vertical position by two plastic plates, each 97 ± 2 mm in diameter and 7.5 to 10.5 mm in thickness, with three holes, 36.0 to 40.6 mm in diameter, equidistant from the center of the plate and equally spaced from one another. Attached to the undersurface of the lower plate is 10-mesh No. 23 (0.025-inch) W. and M. gauge woven stainless-steel wire cloth having a plain square weave. The parts of the apparatus are assembled and rigidly held by means of three bolts passing through the two plastic plates. A suitable means is provided to suspend the basket-rack assembly from the raising and lowering device, using a point on its axis.
The design of the basket-rack assembly may be varied somewhat, provided that the specifications for the glass tubes and the screen mesh size are maintained.
Beaker—
Low form, 1000 mL; the difference between the diameter of the plastic plates, which hold the tubes in a vertical position, and the inside diameter of the beaker should not be more than 6 mm.2
Disks—
Each tube is provided with a perforated cylindrical disk 15.3 ± 0.15 mm thick and 31.4 ± 0.13 mm in diameter. The disk is made of a suitable, transparent plastic material having a specific gravity of between 1.18 and 1.20. Seven 3.15 ± 0.1-mm holes extend between the ends of the cylinder, one of the holes being through the cylinder axis and the others parallel with it and equally spaced on a 4.2-mm radius from it. All surfaces of the disk are smooth.3
Procedure
Uncoated Tablets—
Place 1 tablet in each of the tubes of the basket and, if prescribed, add a disk to each tube. Operate the apparatus, using water or the specified medium as the immersion fluid, maintained at 37 ± 2 . At the end of 30 minutes, lift the basket from the fluid, and observe the tablets: all of the tablets disintegrate completely. If 1 or 2 tablets fail to disintegrate completely, repeat the test on 12 additional tablets. The requirement is met if not fewer than 16 of the total of 18 tablets tested disintegrate completely.
. At the end of 30 minutes, lift the basket from the fluid, and observe the tablets: all of the tablets disintegrate completely. If 1 or 2 tablets fail to disintegrate completely, repeat the test on 12 additional tablets. The requirement is met if not fewer than 16 of the total of 18 tablets tested disintegrate completely.
Plain Coated Tablets—
Place 1 tablet in each of the tubes of the basket and, if the tablet has a soluble external sugar coating, immerse the basket in water at room temperature for 5 minutes. Then, if prescribed, add a disk to each tube, and operate the apparatus, using water or the specified medium as the immersion fluid, maintained at 37 ± 2 . At the end of 30 minutes, lift the basket from the fluid, and observe the tablets: all of the tablets disintegrate completely. If 1 or 2 tablets fail to disintegrate completely, repeat the test on 12 additional tablets. The requirement is met if not fewer than 16 of the total of 18 tablets tested disintegrate completely.
. At the end of 30 minutes, lift the basket from the fluid, and observe the tablets: all of the tablets disintegrate completely. If 1 or 2 tablets fail to disintegrate completely, repeat the test on 12 additional tablets. The requirement is met if not fewer than 16 of the total of 18 tablets tested disintegrate completely.
Delayed-Release (Enteric-Coated) Tablets—
Place 1 tablet in each of the six tubes of the basket, and if the tablet has a soluble external sugar coating, immerse the basket in water at room temperature for 5 minutes. Then operate the apparatus using simulated gastric fluid TS maintained at 37 ± 2 as the immersion fluid. After 1 hour of operation in simulated gastric fluid TS, lift the basket from the fluid, and observe the tablets: the tablets show no evidence of disintegration, cracking, or softening. Operate the apparatus, using simulated intestinal fluid TS, maintained at 37 ± 2
as the immersion fluid. After 1 hour of operation in simulated gastric fluid TS, lift the basket from the fluid, and observe the tablets: the tablets show no evidence of disintegration, cracking, or softening. Operate the apparatus, using simulated intestinal fluid TS, maintained at 37 ± 2 , as the immersion fluid for the time specified in the monograph. Lift the basket from the fluid, and observe the tablets: all of the tablets disintegrate completely. If 1 or 2 tablets fail to disintegrate completely, repeat the test on 12 additional tablets: not fewer than 16 of the total of 18 tablets tested disintegrate completely.
, as the immersion fluid for the time specified in the monograph. Lift the basket from the fluid, and observe the tablets: all of the tablets disintegrate completely. If 1 or 2 tablets fail to disintegrate completely, repeat the test on 12 additional tablets: not fewer than 16 of the total of 18 tablets tested disintegrate completely.
Delayed-Release (Enteric-Coated) Soft Shell Capsules—
Place 1 softgel capsule in each of the six tubes of the basket. Use two baskets for a total of six tubes for Apparatus B. Omit the use of a disk. Operate the apparatus using simulated gastric fluid TS maintained at 37 ± 2 as the immersion fluid. After 1 hour of operation in simulated gastric fluid TS, lift the basket from the fluid and observe the softgels: the softgels show no evidence of disintegration or rupture permitting the escape of the contents. Operate the apparatus with disks, using simulated intestinal fluid TS, maintained at 37 ± 2
as the immersion fluid. After 1 hour of operation in simulated gastric fluid TS, lift the basket from the fluid and observe the softgels: the softgels show no evidence of disintegration or rupture permitting the escape of the contents. Operate the apparatus with disks, using simulated intestinal fluid TS, maintained at 37 ± 2 , as the immersion fluid. Lift the basket from the fluid, and observe the capsules. All the capsules disintegrate completely within 60 minutes. If 1 or 2 capsules fail to disintegrate completely, repeat the test on 12 additional capsules: not fewer than 16 of a total of 18 capsules tested disintegrate completely.
, as the immersion fluid. Lift the basket from the fluid, and observe the capsules. All the capsules disintegrate completely within 60 minutes. If 1 or 2 capsules fail to disintegrate completely, repeat the test on 12 additional capsules: not fewer than 16 of a total of 18 capsules tested disintegrate completely.
Buccal Tablets—
Apply the test for Uncoated Tablets. After 4 hours, lift the basket from the fluid, and observe the tablets: all of the tablets disintegrate completely. If 1 or 2 tablets fail to disintegrate completely, repeat the test on 12 additional tablets: not fewer than 16 of the total of 18 tablets tested disintegrate completely.
Sublingual Tablets—
Apply the test for Uncoated Tablets. At the end of the time limit specified in the individual monograph, all the tablets disintegrate completely. If 1 or 2 tablets fail to disintegrate completely, repeat the test on 12 additional tablets: not fewer than 16 of the total of 18 tablets tested disintegrate completely.
Hard Shell Capsules—
Apply the test for Uncoated Tablets, using as the immersion fluid, maintained at 37 ± 2 , a 0.05 M acetate buffer prepared by mixing 2.99 g of sodium acetate trihydrate and 1.66 mL of glacial acetic acid with water to obtain a 1000-mL solution having a pH of 4.50 ± 0.05. Attach a removable wire cloth, as described under Basket-Rack Assembly, to the surface of the upper plate of the basket-rack assembly. At the end of 30 minutes, lift the basket from the fluid, and observe the capsules: all of the capsules disintegrate except for fragments from the capsule shell. If 1 or 2 capsules fail to disintegrate completely, repeat the test on 12 additional capsules: not fewer than 16 of the total of 18 capsules tested disintegrate completely.
, a 0.05 M acetate buffer prepared by mixing 2.99 g of sodium acetate trihydrate and 1.66 mL of glacial acetic acid with water to obtain a 1000-mL solution having a pH of 4.50 ± 0.05. Attach a removable wire cloth, as described under Basket-Rack Assembly, to the surface of the upper plate of the basket-rack assembly. At the end of 30 minutes, lift the basket from the fluid, and observe the capsules: all of the capsules disintegrate except for fragments from the capsule shell. If 1 or 2 capsules fail to disintegrate completely, repeat the test on 12 additional capsules: not fewer than 16 of the total of 18 capsules tested disintegrate completely.
Soft Shell Capsules—
Proceed as directed under Rupture Test for Soft Shell Capsules.
Use of Disks—
vitamin–mineral dosage forms—
Add a disk to each tube unless otherwise specified in the individual monograph.
botanical dosage forms—
Omit the use of disks unless otherwise specified in the individual monograph.
dietary supplements other than vitamin–mineral and botanical dosage forms—
Omit the use of disks unless otherwise specified in the individual monograph.
note—The use of disks for enteric-coated tablets is not permitted.
RUPTURE TEST FOR SOFT SHELL CAPSULES
Medium:
water; 500 mL.
Apparatus—
Use Apparatus 2 as described under Dissolution  711
711 , operating at 50 rpm.
, operating at 50 rpm.
Time:
15 minutes.
Procedure—
Place 1 capsule in each vessel, and allow the capsule to sink to the bottom of the vessel before starting rotation of the blade. Use sinkers if the capsules float. Observe the capsules, and record the time taken for each capsule shell to rupture.
Tolerances—
The requirements are met if all of the capsules tested rupture in not more than 15 minutes. If 1 or 2 of the capsules rupture in more than 15 but not more than 30 minutes, repeat the test on 12 additional capsules: not more than 2 of the total of 18 capsules tested rupture in more than 15 but not more than 30 minutes. For soft gelatin capsules that do not conform to the above rupture test acceptance criteria, repeat the test with the addition of purified pepsin to the Medium that results in an activity of 750,000 Units or less per 1000 mL.
Change to read:
DISSOLUTION
This test is provided to determine compliance with the Dissolution requirements where stated in the individual monograph for dietary supplements, except where the label states that tablets are to be chewed.
See Dissolution  711
711 for description of apparatus used, Apparatus Suitability Test, and other related information. Of the types of apparatus described in
for description of apparatus used, Apparatus Suitability Test, and other related information. Of the types of apparatus described in  711
711 , use the one specified in the individual monograph.
, use the one specified in the individual monograph.
Official until December 1, 2011
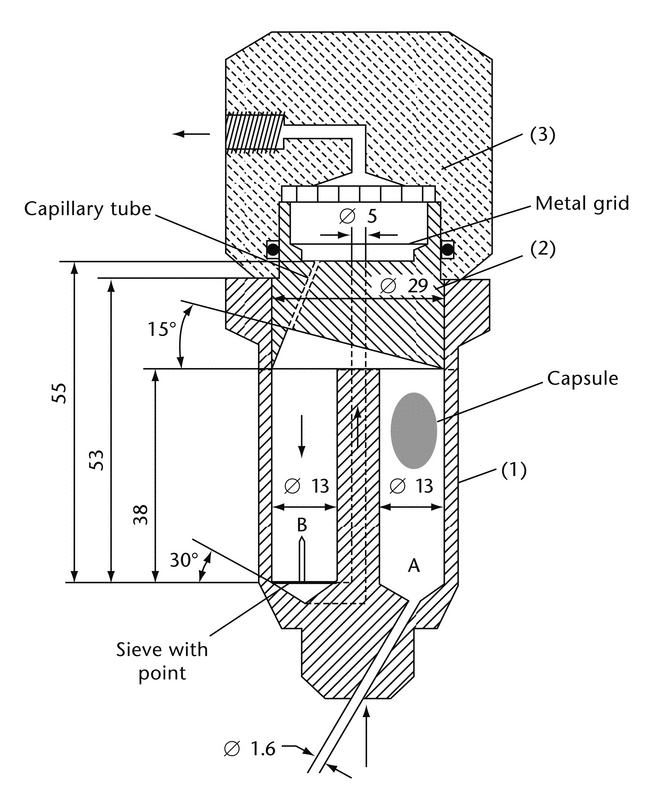
Figure 2 shows the schematic view of a flow-through cell specifically intended for lipid-filled soft gelatin capsules. It consists of three transparent parts that fit into each other (USP Apparatus 4). The lower part (1) is made up of two adjacent chambers connected to an overflow device. The dissolution medium passes through chamber A and is subjected to an upward flow. The flow in chamber B is directed downward to a small-size bore exit that leads upward to a filter assembly. The middle part (2) of the cell has a cavity designed to collect lipophilic excipients that float on the dissolution medium. A metal grid serves as a rough filter. The upper part (3) holds a filter unit for paper, glass fiber, or cellulose filters.
For hard or soft gelatin capsules and gelatin-coated tablets that do not conform to the dissolution specification, repeat the test as follows. Where water or a medium with a pH of less than 6.8 is specified as the Medium in the individual monograph, the same Medium specified may be used with the addition of purified pepsin that results in an activity of 750,000 Units or less per 1000 mL. For media with a pH of 6.8 or greater, pancreatin can be added to produce not more than 1750 USP Units of protease activity per 1000 mL.
This nonspecific dissolution is intended to be diagnostic of known technological problems that may arise as a result of coatings, lubricants, disintegrants, and other substances inherent in the manufacturing process. For dosage forms containing botanical extracts, this dissolution measurement allows an assessment of the extent of decomposition of the extract to polymeric or other nondissoluble compounds that may have been produced by excessive drying or other manipulations involved in the manufacture of botanical extracts. The operative assumption inherent in this procedure is that if the index or marker compound(s) or the extract is demonstrated to have dissolved within the time frame and under conditions specified, the dosage form does not suffer from any of the above formulation or manufacturing related problems.
Vitamin–Mineral Dosage Forms
All dietary supplements belonging to USP Classes II to VI, prepared as tablets or capsules, are subject to the dissolution test and criteria described in this chapter for folic acid (if present) and for index vitamins and index minerals. This test is required because of the importance of the relationship between folate deficiency and the risk of neural tube defects. The accompanying table lists the dissolution requirements for the individual USP classes of dietary supplements. Class I dietary supplements are combinations of oil-soluble vitamins for which dissolution standards are not established; hence, dissolution requirements do not apply to the oil-soluble vitamins contained in formulations belonging to Class IV or Class V. Vitamin–mineral combinations that may not be strictly covered by USP Classes I to Class VI are subject to the dissolution test and criteria specified in the individual monographs.
Dietary Supplements—Vitamin–Mineral
Dosage Forms
Dosage Forms
| USP Class |
Combination of Vitamins or Minerals Present |
Dissolution Requirement |
|---|---|---|
| I | Oil-Soluble Vitamins | Vitamin A (if present)—for tablets only |
| II | Water-Soluble Vitamins | One index vitamin; folic acid (if present) |
| III | Water-Soluble Vitamins with Minerals |
One index vitamin and one index element; folic acid (if present) |
| IV | Oil- and Water-Soluble Vitamins |
One index water-soluble vitamin; folic acid (if present) |
| V | Oil- and Water-Soluble Vitamins with Minerals |
One index water-soluble vitamin and one index element; folic acid (if present) |
| VI | Minerals | One index element |
dissolution conditions for vitamin a tablets
note—Perform this test under light conditions that minimize photo degradation.
Medium:
1% (w/v) sodium ascorbate and 1% (w/v) octoxynol 9 in 0.05 M phosphate buffer pH 6.8; 900 mL
Apparatus 2:
75 rpm
Time:
45 min
dissolution conditions for folic acid
Note—Perform this test under light conditions that minimize photodegradation.
Test 1
Medium:
Water; 900 mL. If the units tested do not meet the requirements for dissolution in water, test 6 additional dosage units for dissolution in a medium of 900 mL of 0.05 M pH 6.0 citrate buffer solution, prepared by mixing 9.5 mL of 0.1 M citric acid monohydrate and 40.5 mL of 0.1 M sodium citrate dihydrate in a 100-mL volumetric flask, diluting with water to volume, mixing, and adjusting to a pH of 6.0 by using either 0.1 M hydrochloric acid or 0.1 M sodium hydroxide solution.
Apparatus 1:
100 rpm, for capsules
Apparatus 2:
75 rpm, for tablets
Time:
1 h
Test 2:
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 2.
Medium:
45 mM citrate buffer, pH 6.0; 250 mL
Apparatus 3:
30 dpm
Screen (Top & Bottom):
56-mesh
Time:
1 h
Note—Compliance with the dissolution requirements for folic acid does not exempt the product from dissolution testing of the pertinent index vitamin or the corresponding index mineral.
dissolution conditions for index water soluble vitamins and index minerals
Test 1
Medium:
0.1 N hydrochloric acid; 900 mL
Apparatus 1:
100 rpm, for capsules
Apparatus 2:
75 rpm, for tablets
Time:
1 h
For formulations containing 25 mg or more of the index vitamin, riboflavin, use the following conditions:
Medium:
0.1 N hydrochloric acid; 1800 mL
Apparatus 1:
100 rpm, for capsules
Apparatus 2:
75 rpm, for tablets
Time:
1 h
Test 2 (Not suitable for Minerals)
If the product complies with this test, the labeling indicates that it meets USP Dissolution Test 2.
Medium:
45 mM citrate buffer, pH 6.0; 250 mL
Apparatus 3:
30 dpm
Screen (Top & Bottom):
56-mesh
Time:
1 h
Note—Compliance with dissolution requirements for the pertinent index vitamin or index mineral does not exempt the product from dissolution testing of folic acid, if present.
selection of index vitamins and index elements
Compliance with the dissolution requirements for dietary supplements representing combinations of water-soluble vitamins (Water-Soluble Vitamins Capsules and Water-Soluble Vitamins Tablets) and combinations of oil- and water-soluble vitamins (Oil- and Water-Soluble Vitamins Capsules and Oil- and Water-Soluble Vitamins Tablets) is determined by measuring the dissolution of a single index vitamin from the water-soluble vitamins present. Riboflavin is the index vitamin when present in the formulation. For formulations that do not contain riboflavin, pyridoxine is the index vitamin. If neither riboflavin nor pyridoxine is present in the formulation, the index vitamin is niacinamide (or niacin), and in the absence of niacinamide (or niacin), the index vitamin is thiamine. If none of the above four water-soluble vitamins is present in the formulation, the index vitamin is ascorbic acid.
Compliance with the dissolution requirements for dietary supplements representing combinations of minerals (Minerals Capsules and Minerals Tablets) is determined by measuring the dissolution of only one index element. Iron is the index element when present in the formulation. For formulations that do not contain iron, the index element is calcium. If neither iron nor calcium is present, the index element is zinc, and in the absence of all three of these elements, magnesium is the index element.
Compliance with the dissolution requirements for dietary supplements representing combinations of water-soluble vitamins and minerals (Water-Soluble Vitamins with Minerals Capsules and Water-Soluble Vitamins with Minerals Tablets) and combinations of oil- and water-soluble vitamins and minerals (Oil- and Water-Soluble Vitamins with Minerals Capsules and Oil- and Water-Soluble Vitamins with Minerals Tablets) is determined by measuring the dissolution of one index water-soluble vitamin and one index element, designated according to the respective hierarchies described above.
procedures
In the following procedures, combine equal volumes of the filtered solutions of the 6 individual specimens withdrawn, and determine the amount of vitamin A, folic acid, or the index vitamin or element dissolved, based on the average of 6 units tested. Make any necessary modifications including concentration of the analyte in the volume of Sample solution taken. Use the Medium for preparation of the Standard solution and dilution, if necessary, of the Sample solution.
Vitamin A:
Determine the percentage of retinyl acetate or retinyl palmitate dissolved by using the following procedure.
Sample solution:
Withdraw a portion of the solution under test, pass through a suitable filter of 0.45-µm pore size, and use the pooled sample as the test specimen.
Standard solution:
Dissolve a suitable amount of USP Retinyl Acetate RS or USP Retinyl Palmitate RS in isopropyl alcohol, and dilute with Medium to obtain a concentration similar to that expected in the Sample solution. [note—The amount of alcohol should be 5%–10%. ]
Solution A:
Methanol and water (90:10)
Solution B:
Methanol and isopropyl alcohol (55:45)
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 8 | 0 | 100 |
| 13 | 0 | 100 |
| 13.1 | 100 | 0 |
| 15 | 100 | 0 |
Chromatographic system
Mode:
LC
Detector:
UV 325 nm
Column:
4.6-mm × 10-cm; 3-µm packing L1
Flow rate:
1.0 mL/min
Injection size:
50 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 1.5 for retinyl acetate and NMT 2.0 for retinyl palmitate
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Result = (rU/rS) × (CS × V/L) × 100
rU = peak area of the all-trans-retinyl ester from the Sample solution
= peak area of the all-trans-retinyl ester from the Sample solution
rS = peak area of the all-trans-retinyl ester from the appropriate Standard solution
= peak area of the all-trans-retinyl ester from the appropriate Standard solution
CS = concentration of retinol (C20H30O) in the appropriate Standard solution (µg/mL)
= concentration of retinol (C20H30O) in the appropriate Standard solution (µg/mL)
V = volume of Medium, 900 mL
= volume of Medium, 900 mL
L = label claim of vitamin A, as retinol (C20H30O) (µg/Tablet)
= label claim of vitamin A, as retinol (C20H30O) (µg/Tablet)
Folic Acid—
Determine the amount of C19H19N7O6 dissolved by using the procedure set forth in the assay for Folic Acid in Oil- and Water-Soluble Vitamins with Minerals Tablets, in comparison with a Standard solution having a known concentration of USP Folic Acid RS in the same Medium.
Niacin or Niacinamide, Pyridoxine, Riboflavin, and Thiamine—
Determine the amount of the designated index vitamin dissolved by using the procedure set forth in the Assay for Niacin or Niacinamide, Pyridoxine Hydrochloride, Riboflavin, and Thiamine in Water-Soluble Vitamins Tablets.
Ascorbic Acid—
Determine the amount of C6H8O6 dissolved by adding 10 mL of 1.0 N sulfuric acid and 3 mL of starch TS to 100.0 mL of sample solution, and titrating immediately with 0.01 N iodine VS. Perform a blank determination, and make any necessary correction.
Iron, Calcium, Magnesium, and Zinc—
Determine the amount of the designated index element dissolved by using the procedure set forth in the appropriate assay in Minerals Capsules.
tolerances
The requirements are met if not less than 75% of the labeled content of vitamin A, not less than 75% of the labeled content of folic acid, and not less than 75% of the labeled content of the index vitamin or the index element from the units tested is dissolved.
Botanical Dosage Forms
Compliance with dissolution requirements necessitates the testing of 6 dosage units individually, or testing 2 or more dosage units in each of the 6 vessels of the dissolution apparatus, and measuring the dissolution of one or more index/marker compound(s) or the extract specified in the individual monograph.
procedures
Combine equal volumes of the filtered solutions of the 6 or more individual specimens withdrawn, and use the pooled sample as the sample solution. Determine the average amount of index or marker compound(s) or the extract dissolved in the pooled sample by the procedure specified in the individual monograph. Make any necessary modifications, including concentration of the analyte in the volume of the sample solution taken. Use the Medium for preparation of the Standard solution and dilution, if necessary, of the sample solution.
tolerances
Unless otherwise specified in the individual monograph, the requirements are met if not less than 75% of the labeled content of the index or marker compound(s) or the extract from the units tested is dissolved in 1 hour.
Dietary Supplements Other Than Vitamin–Mineral and Botanical Dosage Forms
Unless otherwise stated in the individual monographs for dietary supplement dosage forms in this category, compliance requires the testing of 6 individual units, measuring the dissolution of the dietary ingredient as the average of the 6 units tested.
procedures
Combine equal volumes of the filtered solutions of the 6 specimens withdrawn, and use the pooled sample as the Sample solution. Determine the average amount of dietary ingredient dissolved in the pooled sample by the procedure specified in the individual monograph. Make any necessary modifications, including concentration of the analyte in the volume of the Sample solution taken. Use the Medium for preparation of the Standard solution and for dilution, if necessary, of the Sample solution.
tolerances
Because of the diversity of chemical characteristics and solubilities of dietary ingredients pertaining to this category, general tolerances cannot be established. See individual monographs for Tolerances.
1
An apparatus and disks meeting these specifications are available from Varian Inc., 13000 Weston Parkway, Cary, NC 27513, or from laboratory supply houses.
2
1000-mL low-form beakers, designed in compliance with the current ASTM E 960 Type I or Type II or ISO 3819 specifications, meet the size requirements.
3
The use of automatic detection using modified disks is permitted where the use of disks is specified or allowed. Such disks must comply with the requirements for density and dimensions given in this chapter.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Natalia Davydova
Scientific Liaison 1-301-816-8328 |
(GCDF2010) General Chapters - Dosage Forms |
USP35–NF30 Page 972
Pharmacopeial Forum: Volume No. 36(5) Page 1384