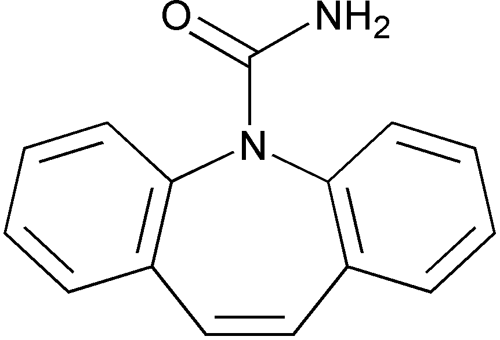
Carbamazepine
(kar'' ba maz' e peen).
5H-Dibenz[b,f]azepine-5-carboxamide.
5H-Dibenz[b,f]azepine-5-carboxamide
» Carbamazepine contains not less than 98.0 percent and not more than 102.0 percent of C15H12N2O, calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
X-ray diffraction  941
941 —
The X-ray diffraction pattern conforms to that of USP Carbamazepine RS, similarly determined.
—
The X-ray diffraction pattern conforms to that of USP Carbamazepine RS, similarly determined.
Acidity—
Add 2.0 g to 40.0 mL of water, mix for 15 minutes, and filter through paper. To a 10.0-mL aliquot of the solution so obtained add 1 drop of phenolphthalein TS, and titrate with 0.01 N sodium hydroxide VS from a 10-mL buret. Perform a blank determination, and make any necessary correction. Not more than 1.0 mL of 0.010 N sodium hydroxide is required for each 1.0 g of Carbamazepine.
Alkalinity—
To a 10.0-mL aliquot of the solution prepared in the test for Acidity add 1 drop of methyl red TS, and titrate with 0.01 N hydrochloric acid VS from a 10-mL buret. Perform a blank determination, and make any necessary correction. Not more than 1.0 mL of 0.010 N hydrochloric acid is required for each 1.0 g of Carbamazepine.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 2 hours: it loses not more than 0.5% of its weight.
for 2 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%, a 2.0-g test specimen being used.
:
not more than 0.1%, a 2.0-g test specimen being used.
Chloride  221
221 —
Boil 1.0 g with 20.0 mL of water for 10 minutes, cool, again adjust the volume, and filter: a 10.0-mL portion of the filtrate shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (0.014%).
—
Boil 1.0 g with 20.0 mL of water for 10 minutes, cool, again adjust the volume, and filter: a 10.0-mL portion of the filtrate shows no more chloride than corresponds to 0.10 mL of 0.020 N hydrochloric acid (0.014%).
Heavy metals, Method II  231
231 :
0.001%.
:
0.001%.
Related compounds—
Mobile phase and System suitability solution—
Proceed as directed in the Assay.
Standard solution—
Dissolve accurately weighed quantities of USP Carbamazepine RS, USP Carbamazepine Related Compound A RS, and USP Carbamazepine Related Compound B RS in methanol, and dilute quantitatively, and stepwise if necessary, with methanol to obtain a solution having known concentrations of about 0.02 mg per mL of each component. Transfer 5.0 mL of this solution to a 100-mL volumetric flask, dilute with a mixture of methanol and water (50:50) to volume, and mix.
Test solution—
Transfer about 100 mg of Carbamazepine, accurately weighed, to a 50-mL volumetric flask, and dissolve in and dilute with methanol to volume. Transfer 25.0 mL of this solution to a 50-mL volumetric flask, add about 20 mL of water, and shake. Allow the mixture to cool to room temperature, and dilute with water to volume.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 230-nm detector and a 4.6-mm × 25-cm column that contains packing L10. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between carbamazepine related compound A and carbamazepine is not less than 1.70; and the relative standard deviation for replicate injections is not more than 2.0%.
)—The liquid chromatograph is equipped with a 230-nm detector and a 4.6-mm × 25-cm column that contains packing L10. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between carbamazepine related compound A and carbamazepine is not less than 1.70; and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the quantities, in mg, of carbamazepine related compound A and carbamazepine related compound B in the portion of Carbamazepine taken by the formula:
100C(ri / rSi)
in which C is the concentration, in mg per mL, of carbamazepine related compound A or carbamazepine related compound B in the Standard solution; and ri and rSi are the peak responses obtained for either carbamazepine related compound A or carbamazepine related compound B from the Test solution and the corresponding peak obtained from the Standard solution, respectively. Calculate the quantities, in mg, of all other impurities found in the portion of Carbamazepine taken by the formula:
100C(ri / rS)
in which ri is the peak response for any other impurity; and rS is the peak response for carbamazepine obtained from the Standard solution: not more than 0.2% of any individual impurity is found; and the total of all impurities (including carbamazepine related compound A and carbamazepine related compound B) is not more than 0.5%.
Assay—
Mobile phase—
Prepare a 1000-mL mixture of water, methanol, and tetrahydrofuran (85:12:3), add 0.22 mL of formic acid, mix, then add 0.5 mL of triethylamine, and mix. Filter and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
System suitability solution—
Dissolve accurately weighed quantities of USP Carbamazepine RS and USP Carbamazepine Related Compound A RS in methanol, and dilute quantitatively, and stepwise if necessary, with methanol to obtain a solution having known concentrations of about 0.1 and 0.5 mg per mL, respectively. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, and dilute with a mixture of methanol and water (1:1) to volume.
Standard preparation—
Dissolve an accurately weighed quantity of USP Carbamazepine RS in methanol, and dilute quantitatively with methanol to obtain a solution having a known concentration of about 2 mg per mL. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, and dilute with a mixture of methanol and water (1:1) to volume.
Assay preparation—
Transfer about 100 mg of Carbamazepine, accurately weighed, to a 50-mL volumetric flask, and dissolve in and dilute with methanol to volume. Transfer 5.0 mL of this solution to a 50-mL volumetric flask, and dissolve in and dilute with a mixture of methanol and water (1:1) to volume.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 230-nm detector and a 4.6-mm × 25-cm column that contains packing L10. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation and the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between carbamazepine related compound A and carbamazepine in the System suitability solution is not less than 1.70; and the relative standard deviation for replicate injections of the Standard preparation is not more than 2.0%.
)—The liquid chromatograph is equipped with a 230-nm detector and a 4.6-mm × 25-cm column that contains packing L10. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation and the System suitability solution, and record the peak responses as directed for Procedure: the resolution, R, between carbamazepine related compound A and carbamazepine in the System suitability solution is not less than 1.70; and the relative standard deviation for replicate injections of the Standard preparation is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. Calculate the quantity, in mg, of C15H12N2O in the portion of Carbamazepine taken by the formula:
500C(rU / rS)
in which C is the concentration, in mg per mL, of USP Carbamazepine RS in the Standard preparation; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison 1-301-816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2481
Pharmacopeial Forum: Volume No. 32(1) Page 65