- British Pharmacopoeia Volume I & II
- Monographs: Medicinal and Pharmaceutical Substances
Ferrous Gluconate |
 |
(Ph Eur monograph 0493)
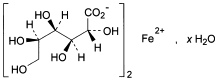
C12H22FeO14,xH2O 446.1 299-29-6
(anhydrous) (anhydrous)
Used in prevention and treatment of iron deficiency.
Ferrous Gluconate contains in 600 mg about 70 mg of iron.
Ph Eur
Iron(II) di(d-gluconate).
11.8 per cent to 12.5 per cent of iron(II) (dried substance). It contains a variable amount of water.
Greenish-yellow or grey powder or granules.
Freely but slowly soluble in water giving a greenish-brown solution, more readily soluble in hot water, practically insoluble in ethanol (96 per cent).
A. Thin-layer chromatography (2.2.27).
Test solution Dissolve 20 mg of the substance to be examined in 2 ml of water R, heating if necessary in a water-bath at 60 °C.
Reference solution Dissolve 20 mg of ferrous gluconate CRS in 2 ml of water R, heating if necessary in a water-bath at 60 °C.
Plate TLC silica gel G plate R.
Mobile phase concentrated ammonia R, ethyl acetate R, water R, ethanol (96 per cent) R (10:10:30:50 V/V/V/V).
Application 5 µl.
Development Over a path of 10 cm.
Drying At 100-105 °C for 20 min.
Detection Allow to cool and spray with a 50 g/l solution of potassium dichromate R in a 40 per cent m/m solution of sulphuric acid R.
Results After 5 min, the principal spot in the chromatogram obtained with the test solution is similar in position, colour and size to the principal spot in the chromatogram obtained with the reference solution.
B. 1 ml of solution S (see Tests) gives reaction (a) of iron (2.3.1).
Dissolve 5.0 g in carbon dioxide-free water R prepared from distilled water R and heated to about 60 °C, allow to cool and dilute to 50 ml with carbon dioxide-free water R prepared from distilled water R.
The solution is clear (2.2.1).
Dilute 2 ml of solution S to 10 ml with water R. Examine the solution against the light.
4.0 to 5.5 for solution S, measured 3-4 h after preparation.
Dissolve 0.5 g in 10 ml of warm water R and add 1 ml of dilute ammonia R1. Pass hydrogen sulphide R through the solution and allow to stand for 30 min. Filter and wash the precipitate with 2 quantities, each of 5 ml, of water R. Acidify the combined filtrate and washings to blue litmus paper R with dilute hydrochloric acid R and add 2 ml in excess. Boil until the vapour no longer darkens lead acetate paper R and continue boiling, if necessary, until the volume is reduced to about 10 ml. Cool, add 15 ml of sodium carbonate solution R, allow to stand for 5 min and filter. Dilute the filtrate to 100 ml with water R. To 5 ml of this solution add 2 ml of cupri-tartaric solution R and boil for 1 min. Allow to stand for 1 min. No red precipitate is formed.
Maximum 0.06 per cent.
Dilute 0.8 ml of solution S to 15 ml with water R.
Dissolve 5.0 g in a mixture of 10 ml of dilute sulphuric acid R and 40 ml of water R. Shake the solution with 50 ml of ether R for 5 min. Separate the aqueous layer and shake it with 20 ml of ether R for 5 min. Combine the ether layers, evaporate to dryness and dissolve the residue in 15 ml of water R. Filter, boil the filtrate until the volume is reduced to 5 ml and add 1 ml of dilute acetic acid R and 1.5 ml of calcium chloride solution R. Allow to stand for 30 min. No precipitate is formed.
Maximum 500 ppm.
To 3.0 ml of solution S add 3 ml of acetic acid R and dilute to 15 ml with distilled water R. Examine the solutions against the light.
Maximum 2 ppm, determined on 0.5 g.
Dilute 10 ml of solution S to 50 ml with distilled water R and add 5 ml of dilute sulphuric acid R. Allow to stand for 5 min. Any opalescence in the solution is not more intense than that in a mixture of 10 ml of solution S and 45 ml of distilled water R.
Maximum 1.0 per cent.
In a ground-glass-stoppered flask, dissolve 5.00 g in a mixture of 10 ml of hydrochloric acid R and 100 ml of carbon dioxide-free water R. Add 3 g of potassium iodide R, close the flask and allow to stand protected from light for 5 min. Titrate with 0.1 M sodium thiosulphate, using 0.5 ml of starch solution R, added towards the end of the titration, as indicator. Carry out a blank titration. Not more than 9.0 ml of 0.1 M sodium thiosulphate is used.
Maximum 20 ppm.
Thoroughly mix 2.5 g with 0.5 g of magnesium oxide R1 in a silica crucible. Ignite to dull redness until a homogeneous mass is obtained. Heat at 800 ± 50 °C for about 1 h, allow to cool and take up the residue in 20 ml of hot hydrochloric acid R. Allow to cool. Transfer the liquid to a separating funnel and shake for 3 min with 3 quantities, each of 20 ml, of methyl isobutyl ketone saturated with hydrochloric acid (prepared by shaking 100 ml of freshly distilled methyl isobutyl ketone R with 1 ml of hydrochloric acid R). Allow to stand, separate the aqueous layer, reduce to half its volume by boiling, allow to cool and dilute to 25 ml with water R. Neutralise 10 ml of this solution to red litmus paper R using dilute ammonia R1 and dilute to 20 ml with water R. 12 ml of the solution complies with test A. Prepare the reference solution using lead standard solution (1 ppm Pb) R.
5.0 per cent to 10.5 per cent, determined on 0.500 g by drying in an oven at 105 °C for 5 h.
TAMC: acceptance criterion 103 CFU/g (2.6.12).
TYMC: acceptance criterion 102 CFU/g (2.6.12).
Dissolve 0.5 g of sodium hydrogen carbonate R in a mixture of 30 ml of dilute sulphuric acid R and 70 ml of water R. When the effervescence stops, dissolve 1.00 g of the substance to be examined with gentle shaking. Using 0.1 ml of ferroin R as indicator, titrate with 0.1 M ammonium and cerium nitrate until the red colour disappears.
1 ml of 0.1 M ammonium and cerium nitrate is equivalent to 5.585 mg of iron(II).
Protected from light.
Ph Eur