Add the following:
(sis'' at ra kure' ee um bes' i late).
C65H82N2O18S2
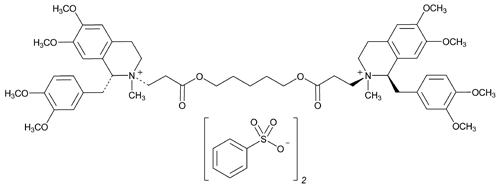
Isoquinolinium, 2,2¢-[1,5-pentanediylbis[oxy(3-oxo-3,1-propanediyl)]]bis[1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-, dibenzenesulfonate, [1R-[1
(1R,2R)-2-(2-Carboxyethyl)-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium benzenesulfonate, pentamethylene ester
DEFINITION
Cisatracurium Besylate contains NLT 97.0% and NMT 102.0% of cisatracurium besylate (C65H82N2O18S2), calculated on the anhydrous and solvent-free basis.
IDENTIFICATION
• B.
ASSAY
• Procedure
Buffer:
Mobile phase:
Diluent:
System suitability solution:
Standard solution:
Sample solution:
Chromatographic system
Mode:
Detector:
Column:
Flow rate:
Injection volume:
Run time:
System suitability
Samples:
[Note—See Table 1 for relative retention times.
Suitability requirements
Resolution:
Tailing factor:
Relative standard deviation:
Analysis
Samples:
Calculate the percentage of cisatracurium besylate (C65H82N2O18S2) in the portion of Cisatracurium Besylate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Cisatracurium Besylate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Cisatracurium Besylate in the Sample solution (mg/mL) |
Acceptance criteria:
IMPURITIES
• Residue on Ignition  281
281 :
:
• Organic Impurities
Mobile phase, Diluent, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability:
Analysis
Sample:
Calculate the percentage of each impurity in the portion of Cisatracurium Besylate taken:
Result = (rU/rT) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rT | = | = sum of all the peak responses from the Sample solution |
Acceptance criteria:
Table 1
| Name |
Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Besylatea
|
0.10 |
— |
| cis-Quaternary acidb
|
0.14 |
0.2 |
| (R)-N-Methyllaudanosinec
|
0.16 |
0.2 |
| (R)-Laudanosined
|
0.20 |
0.6 |
| cis-Quaternary methyl estere
|
0.23 |
0.4 |
| cis-Quaternary alcoholf
|
0.29 |
0.5 |
| R-trans-R¢-trans-Atracuriumg
|
0.74 |
0.2 |
| R-cis-R¢-trans-Atracuriumh
|
0.87 |
0.8 |
| Cisatracurium |
1.0 |
— |
| trans-Monoquaternary compoundi |
1.17 |
0.5 |
| trans-Monoacrylatej
|
1.28 |
0.5 |
| cis-Monoquaternary compoundk |
1.39 |
0.7 |
| cis-cis-Triester analogl
|
1.46 |
0.4 |
| cis-Monoacrylatem
|
1.56 |
1.0 |
| Any individual unspecified impurity |
— |
0.1 |
| Total impurities |
— |
3.0 |
|
a
This peak is due to the counterion and is not to be reported or included in Total impurities.
b
(1R,2R)-2-(2-Carboxyethyl)-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium benzenesulfonate.
c
(R)-1,2,3,4-Tetrahydro-6,7-dimethoxy-2,2-dimethyl-1-veratrylisoquinolinium benzenesulfonate.
d
(R)-1,2,3,4-Tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinoline.
e
(1R,2R)-1,2,3,4-Tetrahydro-6,7-dimethoxy-2-[2-(methoxycarbonyl)ethyl]-2-methyl-1-veratrylisoquinolinium benzenesulfonate.
f
(1R,2R)-1,2,3,4-Tetrahydro-2-(9-hydroxy-3-oxo-4-oxanonyl)-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium benzenesulfonate.
g
(1R,1¢R,2S,2¢S)-2,2¢-(3,11-Dioxo-4,10-dioxatridecamethylene)bis(1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium) dibenzenesulfonate.
h
(1R,1¢R,2R,2¢S)-2,2¢-(3,11-Dioxo-4,10-dioxatridecamethylene)bis(1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium) dibenzenesulfonate.
i
(1R,1¢R,2S)-2-Methyl-2,2¢-(3,11-dioxo-4,10-dioxatridecamethylene)bis(1,2,3,4-tetrahydro-6,7-dimethoxy-1-veratrylisoquinolinium) dibenzenesulfonate.
j
(1R,2S)-2-(3,11-Dioxo-4,10-dioxa-12-tridecenyl)-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium) benzenesulfonate.
k
(1R,1¢R,2R)-2-Methyl-2,2¢-(3,11-dioxo-4,10-dioxatridecamethylene)bis(1,2,3,4-tetrahydro-6,7-dimethoxy-1-veratrylisoquinolinium) dibenzenesulfonate.
l
(1R,1¢R,2R,2¢R)-2,2¢-(3,7,15-Trioxo-4,8,14-trioxa-heptadecamethylene)bis(1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium) dibenzenesulfonate.
m
(1R,2R)-2-(3,11-Dioxo-4,10-dioxa-12-tridecenyl)-(1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium) benzenesulfonate.
|
||
• Limit of Methyl Benzenesulfonate
[Note—Prepare the solutions immediately before use. Methyl benzenesulfonate is slowly hydrolyzed in aqueous solutions.
Mobile phase:
Internal standard solution:
Standard stock solution A:
Standard stock solution B:
Standard solution:
Sample solution:
Chromatographic system
Mode:
Detector:
Column:
Flow rate:
Injection volume:
Run time:
System suitability
Sample:
Suitability requirements
Resolution:
Relative standard deviation:
Analysis
Samples:
Calculate the concentration of methyl benzenesulfonate in the portion of Cisatracurium Besylate taken:
Result = (RU/RS) × (CS/CU)
| RU | = | = peak height ratio of methyl benzenesulfonate to the internal standard from the Sample solution |
| RS | = | = peak height ratio of methyl benzenesulfonate to the internal standard from the Standard solution |
| CS | = | = concentration of methyl benzenesulfonate in the Standard solution (µg/mL) |
| CU | = | = concentration of Cisatracurium Besylate in the Sample solution (g/mL) |
Acceptance criteria:
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781
781
Sample solution:
Acceptance criteria:
 60.0
60.0 to
to  54.0
54.0 at 20
at 20 , calculated on the anhydrous and solvent-free basis
, calculated on the anhydrous and solvent-free basis
• Water Determination, Method Ia  921
921 :
:
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
• USP Reference Standards  11
11
USP Cisatracurium Besylate RS
USP Cisatracurium Besylate System Suitability Mixture RS
Cisatracurium besylate.
R-trans-R¢-trans-Atracurium:
(1R,1¢R,2S,2¢S)-2,2¢-(3,11-Dioxo-4,10-dioxatridecamethylene)bis(1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium) dibenzenesulfonate.
C65H82N2O18S2
R-cis-R¢-trans-Atracurium:
(1R,1¢R,2R,2¢S)-2,2¢-(3,11-Dioxo-4,10-dioxatridecamethylene)bis(1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium) dibenzenesulfonate.
C65H82N2O18S2
Other related compounds.
Cisatracurium besylate.
R-trans-R¢-trans-Atracurium:
(1R,1¢R,2S,2¢S)-2,2¢-(3,11-Dioxo-4,10-dioxatridecamethylene)bis(1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium) dibenzenesulfonate.
C65H82N2O18S2
R-cis-R¢-trans-Atracurium:
(1R,1¢R,2R,2¢S)-2,2¢-(3,11-Dioxo-4,10-dioxatridecamethylene)bis(1,2,3,4-tetrahydro-6,7-dimethoxy-2-methyl-1-veratrylisoquinolinium) dibenzenesulfonate.
C65H82N2O18S2
Other related compounds.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Heather R. Joyce, Ph.D.
Associate Scientific Liaison (301) 998-6792 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP38–NF33 Page 2828
Pharmacopeial Forum: Volume No. 39(5)