INTRODUCTION
For compendial purposes, pH is defined as the value given by a suitable, properly standardized, potentiometric sensor and measuring system. [Note—The measuring system has traditionally been referred to as the “pH meter”. While the pH meter is still in common use, the measuring system can also be embedded inside the pH sensor, and the pH signal can be transmitted digitally to an external device such as a computer, Programmable Logic Controller (PLC), Distributed Control System (DCS), data acquisition system, terminal, or other microprocessor-controlled device. ] By definition, pH is equal to 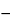 log10[aH+] where aH+ is the activity of the hydrogen (H+) or hydronium ion (H3O+), and the hydrogen ion activity very closely approximates the hydrogen ion concentration.
log10[aH+] where aH+ is the activity of the hydrogen (H+) or hydronium ion (H3O+), and the hydrogen ion activity very closely approximates the hydrogen ion concentration.
The practical pH scale is defined:
pH = pHs + [(E 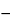 ES)/k]
ES)/k]
E = measured potential where the galvanic cell contains the solution under test (pH)
ES = measured potential where the galvanic cell contains the appropriate buffer solution for standardization (pHs)
k = change in potential per unit change in pH and is derived from the Nernst equation (as follows)
k = loge(10) × (RT/nF)
R = 8.314 J/mole/
T = temperature (
n = moles per half-reaction
F = Faraday constant, 96485 C/mole
The resulting equation is [0.05916 + 0.0001984(T 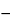 25
25 )] volts at temperature T. Values of k from 15
)] volts at temperature T. Values of k from 15 –35
–35 are provided in Table 1.
are provided in Table 1.
Table 1. Values of k for Various Temperatures
| Temperature ( |
k
(V) |
|---|---|
| 15.00 | 0.05718 |
| 20.00 | 0.05817 |
| 25.00 | 0.05916 |
| 30.00 | 0.06016 |
| 35.00 | 0.06115 |
Values of k at other temperatures can be determined from the equation above. For practical purposes, values of k are determined from pH sensor calibration.
pH MEASUREMENT SYSTEM
The measurement system consists of: (1) a measuring electrode sensitive to hydrogen-ion activity, typically a glass electrode, though other electrode types are possible, (2) a suitable reference electrode, for example, a silver–silver chloride electrode, and (3) a voltage measurement system with an input resistance capable of measuring at a high input impedance of the pH sensor. The measuring and reference electrode may be separated or combined. The voltage measurement system may be separated from the pH sensor or integrated into the sensor. For most applications, a temperature measurement will be necessary for compensation of the Nernst temperature influence described above. A temperature device may be embedded into the pH sensor, or an external temperature device may be used.
INSTRUMENT REQUIREMENTS
The measurement system shall be capable of performing a 2-point pH calibration (see below). The accuracy of the pH measurement system is described in the Calibration section. The resolution of the pH measurement system shall be at least 0.01 pH. The instrument shall be capable of temperature-compensating the pH sensor measurement to convert the millivolt signal to pH units at any temperature, either automatically using a temperature device built into the sensor system or by manual entry of the sample temperature into the measurement system. The accuracy of the temperature measurement system shall be ±1 C. The resolution of the temperature measurement system shall be at least 0.1
C. The resolution of the temperature measurement system shall be at least 0.1 C. Lab-based pH measurements are typically performed at 25 ± 2
C. Lab-based pH measurements are typically performed at 25 ± 2 C unless otherwise specified in the individual monograph or herein. However, temperatures outside this range are acceptable if samples are more conveniently prepared at alternative temperatures. Examples of non-lab-based measurements include test samples inside process pipes, vessels, tanks, and other non-standard processing conditions. [Note—The definitions of pH, the pH scale, and the values assigned to the buffer solutions for standardization are for the purpose of establishing a practical, operational system so that results may be compared between laboratories. The measured pH values do not necessarily correspond exactly to those obtained by the definition, pH =
C unless otherwise specified in the individual monograph or herein. However, temperatures outside this range are acceptable if samples are more conveniently prepared at alternative temperatures. Examples of non-lab-based measurements include test samples inside process pipes, vessels, tanks, and other non-standard processing conditions. [Note—The definitions of pH, the pH scale, and the values assigned to the buffer solutions for standardization are for the purpose of establishing a practical, operational system so that results may be compared between laboratories. The measured pH values do not necessarily correspond exactly to those obtained by the definition, pH = 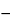 log aH+, rather the values obtained are closely related to the activity of the hydrogen-ion in aqueous solutions. ] [Note—Where a pH measurement system is standardized by use of an aqueous buffer and then used to measure the “pH” of nonaqueous systems, the ionization constant of the acid or base, the dielectric constant of the medium, the liquid-junction potential (which may give rise to errors of approximately 1 pH unit), and the hydrogen-ion response of the glass electrode are all changed. For these reasons, the values so obtained with solutions that are only partially aqueous in character can be regarded only as apparent pH values. ]
log aH+, rather the values obtained are closely related to the activity of the hydrogen-ion in aqueous solutions. ] [Note—Where a pH measurement system is standardized by use of an aqueous buffer and then used to measure the “pH” of nonaqueous systems, the ionization constant of the acid or base, the dielectric constant of the medium, the liquid-junction potential (which may give rise to errors of approximately 1 pH unit), and the hydrogen-ion response of the glass electrode are all changed. For these reasons, the values so obtained with solutions that are only partially aqueous in character can be regarded only as apparent pH values. ]
BUFFER SOLUTIONS FOR STANDARDIZATION OF THE pH MEASUREMENT SYSTEM
Buffer solutions for standardization are prepared as directed in Table 2.1 Buffer salts of requisite purity can be obtained from the National Institute of Standards and Technology, other national authorities, or other suppliers. Buffer solutions should be stored in appropriate containers that ensure stability of the pH through the expiry date, and fitted with a tight closure. For buffer solutions greater than 11, the storage should be in containers that are resistant to or reduce carbon dioxide intrusion which would lower the pH of the buffer. For buffer solutions lower than 11, they should typically be prepared at intervals not to exceed 3 months. For buffer solutions greater than 11, they should typically be prepared and used fresh unless carbon dioxide ingress is restricted. All buffer solutions should be prepared using Purified Water. Table 2 indicates the pH of the buffer solutions as a function of temperature. The instructions presented here are intended for the preparation of solutions having the designated molal (m) concentrations. However, in order to facilitate their preparation, the instructions are given in molarity. The difference in concentration between molality and molarity preparations for these buffer solutions is less than 1%, and the pH difference is negligible. Calibration using buffer solutions shall be done in the temperature range of the buffers listed in Table 2. [Note—The Nernst temperature compensation corrects only for the electrode millivolt output change with temperature, not the actual pH change of the buffer solution with temperature which is unique for each buffer. ] Features such as automatic buffer recognition or buffer pH–temperature correction are available for convenience in accommodating the temperature influence on buffer solutions. The pH–temperature response can be determined from the values in Table 2.
Table 2. pH Values of Buffer Solutions for Standardization
| Temper- ature ( |
Potassium Tetra- oxalate, 0.05 m |
Potassium Hydrogen Tartrate Saturated at 25 |
Potassium Dihydrogen Citrate, 0.05 M |
Potassium Biphthalate, 0.05 m |
Equimolal Phosphate, 0.05 m |
Potassium Dihydrogen Phosphate, 0.0087 M, and Disodium Hydrogen Phosphate, 0.0303 M |
Sodium Tetraborate, 0.01 m |
Sodium Carbonate, 0.025 M, and Sodium Bicarbonate, 0.025 M |
Calcium Hydroxide, Saturated at 25 |
|---|---|---|---|---|---|---|---|---|---|
| 10 | 1.67 | — | — | 4.00 | 6.92 | — | 9.33 | — | 13.00 |
| 15 | 1.67 | — | 3.80 | 4.00 | 6.90 | 7.45 | 9.28 | 10.12 | 12.81 |
| 20 | 1.68 | — | 3.79 | 4.00 | 6.88 | 7.43 | 9.23 | 10.06 | 12.63 |
| 25 | 1.68 | 3.56 | 3.78 | 4.01 | 6.86 | 7.41 | 9.18 | 10.01 | 12.45 |
| 30 | 1.68 | 3.55 | 3.77 | 4.02 | 6.85 | 7.40 | 9.14 | 9.97 | 12.29 |
| 35 | 1.69 | 3.55 | 3.76 | 4.02 | 6.84 | 7.39 | 9.10 | 9.93 | 12.13 |
| 40 | 1.69 | — | — | 4.04 | 6.84 | — | 9.07 | — | 11.98 |
| 45 | 1.70 | — | — | 4.05 | 6.83 | — | 9.04 | — | 11.84 |
| 50 | 1.71 | — | — | 4.06 | 6.83 | — | 9.01 | — | 11.71 |
| 55 | 1.72 | — | — | 4.08 | 6.83 | — | 8.99 | — | 11.57 |
| 60 | 1.72 | — | — | 4.09 | 6.84 | — | 8.96 | — | 11.45 |
| DpH/D |
0.0010 | 0.0018 |
Preparation of alternative volumes at the same concentrations to those indicated below is acceptable.
Potassium tetraoxalate, 0.05 m:
Dissolve 12.61 g of KH3(C2O4)2·2H2O, and dilute with water to make 1000.0 mL.
Potassium hydrogen tartrate, saturated at 25 :
Add C4H5KO6 to water until saturation is exceeded at 25
:
Add C4H5KO6 to water until saturation is exceeded at 25 . Then filter or decant.
. Then filter or decant.
Potassium dihydrogen citrate, 0.05 M:
Dissolve 11.41 g C6H7KO4, and dilute with water to make 1000.0 mL.
Potassium biphthalate, 0.05 m:
Dissolve 10.12 g of KHC8H4O4, previously dried at 110 for 1 h, and dilute with water to make 1000.0 mL.
for 1 h, and dilute with water to make 1000.0 mL.
Equimolal phosphate, 0.05 m:
Dissolve 3.53 g of Na2HPO4 and 3.39 g of KH2PO4, each previously dried at 120 for 2 h, and dilute with water to make 1000.0 mL.
for 2 h, and dilute with water to make 1000.0 mL.
Potassium dihydrogen phosphate, 0.0087 M, and disodium hydrogen phosphate, 0.0303 M:
Dissolve 1.18 g of KH2PO4 and 4.30 g Na2HPO4, both dried for 2 h at 120 ± 2 , and dilute with water to make 1000.0 mL.
, and dilute with water to make 1000.0 mL.
Sodium tetraborate, 0.01 m:
Dissolve 3.80 g of Na2B4O7·10H2O, and dilute with water to make 1000.0 mL. Protect from absorption of carbon dioxide.
Sodium carbonate, 0.025 M, and sodium bicarbonate, 0.025 M:
Dissolve 2.64 g of Na2CO3 and 2.09 g NaHCO3, and dilute with water to make 1000.0 mL.
Calcium hydroxide, saturated at 25 :
Add Ca(OH)2 to water until saturation is exceeded at 25
:
Add Ca(OH)2 to water until saturation is exceeded at 25 . Use water that has been recently boiled and protected from atmosphere to limit carbon dioxide absorption. Then filter or decant.
. Use water that has been recently boiled and protected from atmosphere to limit carbon dioxide absorption. Then filter or decant.
CALIBRATION
Because of variations in the nature and operation of the available pH measurement systems, it is not practical to provide universal directions for the standardization of the measurement system. However, the general principles to be followed are set forth in the following paragraphs. Examine the electrodes, especially the reference electrode and electrolyte level, if a liquid electrolyte is used. If necessary, replenish electrolyte supply, and observe other precautions indicated by the instrument and electrode manufacturers.
The calibration or verification of the pH measurement system should be periodically executed. The historical performance of the measurement system, the criticality of the pH measurement, the maintenance of the pH sensor, and the frequency of measurement operation is used to determine the frequency of the calibration/verification.
If the pH of the buffer is sensitive to ambient carbon dioxide, then use Purified Water that has been recently boiled, and subsequently stored in a container designed to minimize ingress of carbon dioxide.
- To standardize the pH measurement system, select three buffer solutions for standardization, preferably from those given in Table 2, such that the expected pH of the material under test falls within their range. Two of the buffers are used for the calibration process, and the third buffer is used for verification. The value of the verification buffer should be between the calibration buffers. If the operational range of the pH sensor is beyond the pH range of the buffer solutions in Table 2, then either 1) select two nearby pH buffers from Table 2 or 2) select one from Table 2 and another documented prepared buffer that is outside the range.
- Rinse the pH sensor several times with water, then with the first buffer solution.
- Immerse the pH sensor in the first buffer solution at a temperature within the range of Table 2.
- If automatic temperature measurement and compensation is not included in the measuring system, manually enter the temperature of the buffer and pH value of the buffer solution at that temperature into the instrument. For temperatures not listed in Table 2, use linear interpolation to determine the pH value as a function of temperature.
- Initiate the 2-point calibration sequence with the first buffer according to manufacturer's instructions.
- Remove the pH sensor from the first buffer and rinse the electrode(s) with water, and then the second buffer solution.
- Immerse the pH sensor in the second buffer at a temperature within the range of Table 2.
- If automatic temperature measurement and compensation is not included in the measuring system, manually enter the temperature of the buffer and pH value of the buffer solution at that temperature into the instrument.
- Continue the 2-point calibration sequence with the second buffer according to manufacturer¢s instructions.
-
After completion of the 2-point calibration process, verify that the pH slope and offset are within acceptable parameters. Typical acceptable parameters are slopes ranging from 90%–105% and an offset of ±30 mV (0.5 pH units at 25
 C). Depending on the pH instrumentation, the pH slope and offset may be determined in software or by manual methods. If these parameters are not within acceptable parameters, the sensor should be properly cleaned, replenished, serviced, or replaced, and the 2-point calibration process shall be repeated.
C). Depending on the pH instrumentation, the pH slope and offset may be determined in software or by manual methods. If these parameters are not within acceptable parameters, the sensor should be properly cleaned, replenished, serviced, or replaced, and the 2-point calibration process shall be repeated.
- Remove the pH sensor from the second buffer, and rinse thoroughly with water, and then the verification buffer.
- Immerse the pH sensor in the verification buffer at a temperature within the range of Table 2.
- If automatic temperature measurement and compensation is not included in the measuring system, manually enter the temperature of the buffer and pH value of the buffer solution at that temperature into the instrument.
- The pH reading shall be within ±0.05 pH of the value in Table 2 at the buffer solution temperature.
OPERATION
All test samples should be prepared using Purified Water, unless otherwise specified in the monograph. All test measurements should use manual or automated Nernst temperature compensation.
- Prepare the test material according to requirements in the monograph or according to specific procedures. If the pH of the test sample is sensitive to ambient carbon dioxide, then use Purified Water that has been recently boiled, and subsequently stored in a container designed to minimize ingress of carbon dioxide.
- Rinse the pH sensor with water, then with a few portions of the test material.
- Immerse the pH sensor into the test material and read the pH value and temperature.
In all pH measurements, allow sufficient time for stabilization of the temperature and pH measurement.
Diagnostic functions such as glass or reference electrode resistance measurement may be available to determine equipment deficiencies. Refer to electrode supplier for diagnostic tools to assure proper electrode function.
Where approximate pH values suffice, indicators and test papers (see Indicators and Indicator Test Papers, in the section Reagents, Indicators, and Solutions) may be suitable.
For a discussion of buffers, and for the composition of standard buffer solutions called for in compendial tests and assays, see Buffer Solutions in the section Solutions. This referenced section is not intended to replace the use of the pH calibration buffers in Table 2.
1
Commercially available buffer solutions for pH measurement system, standardized by methods traceable to the National Institute of Standards and Technology (NIST) or other national authorities, labeled with a pH value accurate to 0.02 pH unit may be used. Solutions prepared from ACS reagent grade materials or other suitable materials, may be used provided the pH of the resultant solution is the same as that of the solution prepared from the NIST (or other national authorities) certified material. Buffer solutions that are greater than 12 should be used immediately, or should be prepared using freshly boiled water, and stored under conditions to minimize carbon dioxide absorption and ingress.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Antonio Hernandez-Cardoso, M.Sc.
Senior Scientific Liaison (301) 816-8308 |
(GCCA2010) General Chapters - Chemical Analysis |
USP38–NF33 Page 556
Pharmacopeial Forum: Volume No. 39(5)



