Add the following:
INTRODUCTION
Fluorescence is a two-step process that requires absorption of light at a specific wavelength (excitation) followed by emission of light, usually at a higher wavelength. The emission of light is termed fluorescence.
The most common type of fluorescent sample is a submicromolar transparent solution that absorbs light following the Beer–Lambert–Bouguer Law and fluoresces with an intensity that is directly proportional to the concentration, the absorptivity, and the fluorescence quantum yield of the fluorescent species or fluorophore.
Unlike absorption spectroscopy, where deviation from linearity is the exception, fluorescence linearity can be affected by a number of sample-related effects. For additional information, see Fluorescence Spectroscopy—Theory and Practice  1853
1853 .
.
Fluorescence methods also are termed background-free because little excitation light reaches the detector. This characteristic makes fluorescence detection highly sensitive, down to single-molecule detection in some cases. Fluorescence detection also can be highly specific because a fluorophore emits a characteristic emission pattern. Specificity and sensitivity are two of the more important strengths of fluorescence methods.
QUALIFICATION OF FLUORESCENCE INSTRUMENTS
Analysts ensure the suitability of a specific instrument for a given procedure by using a stepwise evaluation for the desired application from selection to instrument retirement: design qualification (DQ); installation qualification (IQ); an initial performance-to-specification qualification, also known as operational qualification (OQ); and an ongoing performance qualification (PQ). For additional information, see general chapter Analytical Instrument Qualification  1058
1058 .
.
DQ and IQ are not further considered in this chapter. The purpose of this section is to provide test methods and acceptance criteria to ensure that the instrument is suitable for its intended use (OQ) and that it will continue to function properly over extended time periods (PQ).
As with any spectrometric device, analysts must qualify a spectrofluorometer for both wavelength (x-axis) and relative intensity (y-axis or signal axis) accuracy and precision. They also must establish sensitivity. OQ should span the operational ranges required within the laboratory for both intensity and wavelength scales.
Instrument Operational Qualification
The tolerances given in both the instrument OQ and PQ are applicable for general use. Specifications for particular instruments and applications can vary depending on the analytical procedure used and the desired accuracy of the final result. Instrument vendors often have samples and test parameters available as part of the IQ/OQ package.
Wherever possible, analysts should use certified reference materials for purposes of calibration in the steps detailed below in preference to laboratory-prepared solutions. When certified reference materials are obtained from a recognized accredited source, they have independently verified traceable value assignments with associated calculated uncertainties.
Two general types of instrumental measurements are differentiated here: spectral (i.e., those that measure intensity versus wavelength) and fixed (i.e., those that measure intensity at a fixed wavelength and bandwidth).
control of wavelengths
The level of confidence of measured peak positions is defined by wavelength accuracy for spectral measurements. Determination of the accuracy of many wavelengths across the desired wavelength range demonstrates if further calibration beyond a single point is needed. Multipoint calibration involves measuring wavelength biases at multiple wavelengths and correcting for the wavelength dependence of the bias. A single-point calibration often can be applied to the wavelength axis in an instrument's software before data are collected, but a multipoint calibration may require that the correction be applied to spectra after they are collected.
For fixed measurements, the wavelength position and bandwidth must be reproducible. For filter-based wavelength selection, this requires that only the same filter be used when analysts compare data over time. If a different filter must be used (e.g., when data are compared across instruments and laboratories), then the transmission curves of the filters must be compared.
Wavelength precision should be determined over the operational range using at least six replicate measurements. The standard deviation should not exceed ±1 nm.
atomic line spectra
This procedure is described as the primary application because the emission lines produced from a discharge lamp are characteristic of the source element, and, as a fundamental physical standard, these wavelengths have been measured with an uncertainty of NMT ± 0.01 nm. In solution spectrofluorometry the wavelength bias required rarely exceeds 1.0 nm. For these reasons, the atomic line standard values are cited without uncertainty. The lamp should be placed at the source position in the spectrofluorometer.
A commonly employed low-pressure mercury lamp has a number of intense lines that cover a large part of the UV and visible range. Manufacturers often use two Xenon lines from the source at 260.5 and 541.9 nm as an internal calibration check, because the accuracy of both the excitation and emission monochromators can be verified and can be used for diagnostic purposes (see Table 1).1
Table 1. Elemental Line Spectra Wavelengths
| Element |
Wavelength (nm) |
|---|---|
| Hg |
253.7 |
| Xe |
260.5 |
| Hg |
296.7 |
| Hg |
365.0 |
| Hg |
404.7 |
| Hg |
435.8 |
| Xe |
541.9 |
| Hg |
546.1 |
| Hg |
577.0 |
| Hg |
579.1 |
use of rare earth oxide solutions
This procedure uses a solution of a rare earth oxide prepared by dissolution in acid media. The most frequently used is holmium oxide in perchloric acid in combination with a diffuse reflector located at the sample position. Suitable certified reference materials are available commercially.2 The wavelength selector not being scanned should be removed; if removal is not practicable, it should be set to zero order (in this position a grating behaves like a mirror reflecting all wavelengths). The diffuse reflector is scanned with and without the rare earth sample in place, and the ratio of the two intensities is calculated to obtain an effective transmittance spectrum. Minima in the intensity ratio correspond to absorption peaks of the sample. For a 4% (w/w) solution of holmium oxide in perchloric acid at 1.0 nm spectral bandwidth and a path length of 1 cm, these minima are in Table 2.3
Table 2
| Wavelength (nm) |
|---|
| 241.1 |
| 249.9 |
| 278.1 |
| 287.2 |
| 333.5 |
| 345.4 |
| 361.3 |
| 385.6 |
| 416.3 |
| 451.4 |
| 467.8 |
| 485.2 |
| 536.6 |
| 640.5 |
If the operational range of the spectrophotometer lies outside 240–650 nm, other certified rare earth oxides or other solutions are used.
Didymium (a mixture of neodymium and praseodymium) is available as a traceable standard in both solution and glass presentations. Didymium is similar in preparation to the holmium materials and has useful peak characteristics in the 730–870 nm region. Useful peaks are found in the didymium solution at approximately 731.6, 740.0, 794.1, 799.0, and 864.4 nm.
use of polymethylmethacrylate-doped references
This procedure uses solid reference materials manufactured by polymerization of a variety of fluorescent active aromatic ring compounds into an inert polymethylmethacrylate (PMMA) matrix. These materials are supplied as polished blocks for use in a standard cuvette holder (see Table 3).
Table 3. Dopant, Excitation, and Emission Data for Selected Reference Materials
| Dopant |
Excitation (nm) |
Emission (nm) |
|---|---|---|
| p-Terphenyl |
295 |
338 |
| Ovalene |
342 |
482 |
| Tetraphenylbutadiene |
348 |
422 |
| Anthracene |
360 |
402 |
Performance Verification
Results from day-to-day testing of photostable intensity standards are used to verify the performance of an instrument. If the measured intensity does not change from that observed when the instrument was qualified, then instrument performance has not changed and remains qualified. Using such standards to determine an artifact-based or quasi-absolute intensity scale potentially enables measured intensities and instrument sensitivity to be compared over time or between instruments. Intensity measurements should be within the linear range of the instrument's detection system before analysts attempt intensity comparisons, and will be affected by any instrumental effect directly related to the fluorescence signal, e.g., changes in source intensity, detector response, etc.
For instruments with filter-based wavelength selection, analysts use fluorescence standards for spectral correction to determine expected intensity differences caused by filters with different transmission profiles. By compensating for these intensity differences due to spectral mismatch, the analyst can determine a quasi-absolute intensity scale for these instruments. Analysts should approach instrument-to-instrument comparisons with particular caution because of the relatively large and difficult-to-quantify uncertainties involved.
use of low-conductivity (18-MW) water
The Raman band of water is used to measure signal-to-noise ratios in fluorescent instruments. The Raman band of water is inherently reproducible and does not degrade with time. Water is convenient to obtain in a pure state and allows interlaboratory comparisons to be made with a high level of confidence. No preparation or dilution is required. The Raman band is a low-level signal that provides a good test for both the optics and the electronics of an instrumental system.
The Raman band of water is not caused by fluorescence but is a result of Raman scattering. For water, the Raman band is always red-shifted 3382 cm–1 relative to the excitation.4 This band usually is measured by excitation at 350 nm, resulting in a Raman peak at 397 nm, but radiation up to 500 nm also can be used as the excitation wavelength, and the corresponding emission peak is 602 nm.
use of intensity standards
Several solid-doped fluorescent materials are available.5 These polymers or glasses enable the relative spectral correction and day-to-day performance qualification of fluorescence instruments across the UV, visible, and NIR regions from 320 to 830 nm. The high photostability of the materials makes them particularly useful as day-to-day intensity standards, even when spectral correction is not needed or when the excitation wavelength differs from that used for certification. A certified, steady-state emission spectrum is supplied with each certified reference material, along with the estimated total uncertainties. The reference is available in the form of a solid glass, standard-sized cuvette (12.5 mm × 12.5 mm × 45 mm) with three polished long faces for 90 detection and one frosted long face for front-face or epifluorescence detection.
detection and one frosted long face for front-face or epifluorescence detection.
QUALITATIVE AND QUANTITATIVE FLUORESCENCE MEASUREMENTS
Two general classes of procedural measurements commonly are performed by fluorescence spectrometry: qualitative and quantitative.
Qualitative Fluorescence Measurements
Qualitative fluorescence measurements are used to detect the presence of particular analytes and yield a positive or negative answer. The excitation and emission wavelengths often are selected at the peak maximum of the fluorophore to be detected. The minimum amount of analyte needed for a positive result should be considered by the analyst to ensure that the method is appropriate for the particular application. The observation of fluorescence at the peak position above the limit of detection (usually 3 times the noise level) indicates a positive result.
Quantitative Fluorescence Measurements
Quantitative fluorescence measurements are used to determine amounts or concentrations of analytes in unknown samples. These quantities may be determined in absolute units, such as moles or moles per L, or in relative units, such as the ratio of the concentrations of two fluorescent analytes contained in a single unknown solution. These determinations use the following proportionality relating fluorescence signal (S) at a given pair of excitation and emission wavelengths (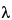 ex,
ex, 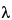 em) to fluorescent analyte concentration (c):
em) to fluorescent analyte concentration (c):
S 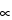 /0 × W × Rd ×
/0 × W × Rd ×  × F × c
× F × c
/0 = intensity of the excitation beam
W = fraction of the fluorescence collected by the detection system
Rd = responsivity of the detection system
F = fluorescence quantum yield
c = concentration of the fluorescent analyte
This linear proportionality with concentration applies to optically dilute samples (e.g., solutions with an absorbance of less than 0.05 at a path length of 1 cm).
GOOD SPECTROSCOPIC PRACTICE
Comparisons of a test specimen with a Reference Standard are best made at a peak of spectral emission for the compound of interest. Assays based on spectrofluorometry give the commonly accepted wavelengths for excitation and peak spectral emission of the substance in question. Different spectrofluorometers may show minor variation in the apparent wavelength of this peak. Comparisons should be made at the wavelength at which peak emission occurs. If this differs from the wavelength specified in the monograph by more than ±1 nm in the range of 200–400 nm or by more than ±2 nm in the range of 400–800 nm, recalibration of the instrument may be indicated.
Use of Reference Standards
With few exceptions, pharmacopeial spectrofluorometric procedures provide results by comparison against a USP Reference Standard. This ensures measurement under identical conditions for the test specimen and the Reference Standard. These conditions could include wavelength setting, spectral bandwidth selection, cell placement and correction, and intensity levels. Cells that exhibit identical optical fluorescence characteristics at a specific wavelength may differ considerably at other wavelengths. Analysts should establish and use appropriate cell corrections where required.
The terms similar preparation and similar solution in tests and assays that involve spectrofluorometry indicate that the Reference Standard should be prepared and observed in a manner that is identical to that used for the sample under test. Usually when a solution of the specified Reference Standard is prepared at (i.e., within 10% of) the desired concentration, the fluorescence intensity is calculated on the basis of the exact amount weighed out. If analysts have not used a previously dried specimen of the Reference Standard, they should correct this intensity on the anhydrous basis.
The expressions concomitantly determine and concomitantly measured as used in procedures that involve spectrofluorometry indicate that the fluorescence of both the sample solution and the standard solution, relative to the specified test blank, are to be measured in immediate succession.
Sample Solution Preparation
For determinations using UV or visible spectrofluorometry, the specimen generally is dissolved in a solvent. Unless otherwise directed in the monograph, determinations are made at room temperature by using a path length of 1 cm. Many solvents are suitable for these ranges, including water, alcohols, chloroform, lower hydrocarbons, ethers, and dilute solutions of strong acids and alkalis. Solvents should be free from contaminants that fluoresce in the spectral region under examination. For the solvent, water-free methanol or alcohol or alcohol that has been denatured by the addition of methanol but does not contain benzene or other interfering impurities should be used. Spectrophotometric-quality solvents that are guaranteed to be free from contaminants are available commercially from several sources, but some analytical reagent-grade organic solvents may contain traces of impurities that fluoresce strongly in the UV region. New lots of these solvents should be checked for their transparency, and analysts should take care to use the same lot of solvent for the preparation of the sample solution, the standard solution, and the blank. Solvents that do not have an interfering fluorescence signature at the wavelength(s) of interest should be used. In normal usage, the fluorescence baseline intensity should not be more than 2% of the expected measurement signal unless a larger value previously has been justified.
Assays in the visible region usually call for comparing concomitantly the fluorescence intensities produced by the sample solution with that produced by a standard solution that contains approximately an equal quantity of a USP Reference Standard. In some situations, it may be permissible to omit the use of a Reference Standard. This is true when spectrofluorometric assays are made with routine frequency, when a suitable standard curve is available and is prepared with the appropriate USP Reference Standard, and when the substance assayed conforms to Beer–Lambert–Bouguer Law within the range of about 75%–125% of the final concentration used in the assay. Under these circumstances, the fluorescence intensity found in the assay can be interpolated on the standard curve, and the assay result can be calculated. Such standard curves should be confirmed frequently and always when a new spectrofluorometer or new lots of reagents are put into use.
VALIDATION AND VERIFICATION
Validation
Validation is required when a procedure based on fluorescence spectroscopy is intended for use as an alternative to the official procedure. The objective of validation is to demonstrate that the measurement is suitable for its intended purpose, including the following: quantitative determination of the main component in a drug substance or a drug product (Category I assays), quantitative determination of impurities or limit tests (Category II), and identification tests (Category IV). Depending on the category of the test (for additional information, see Table 2 in Validation of Compendial Procedures  1225
1225 ), the process for analytical procedure validation for fluorescence requires testing for linearity, range, accuracy, specificity, precision, detection limit, quantitation limit, and robustness. These analytical performance characteristics apply to externally standardized procedures and those that use standard additions. Chapter
), the process for analytical procedure validation for fluorescence requires testing for linearity, range, accuracy, specificity, precision, detection limit, quantitation limit, and robustness. These analytical performance characteristics apply to externally standardized procedures and those that use standard additions. Chapter  1225
1225 provides definitions and general guidance about analytical procedures validation without indicating specific validation criteria for each characteristic. The intention of the following sections is to provide the user with specific validation criteria that represent the minimum expectations for fluorescence technology. For each particular application tighter criteria may be needed in order to demonstrate suitability for the intended use.
provides definitions and general guidance about analytical procedures validation without indicating specific validation criteria for each characteristic. The intention of the following sections is to provide the user with specific validation criteria that represent the minimum expectations for fluorescence technology. For each particular application tighter criteria may be needed in order to demonstrate suitability for the intended use.
accuracy
For Category I, Category II, and Category III procedures, accuracy is determined by conducting recovery studies with the appropriate matrix spiked with known concentrations of the analyte. Analysts also can compare assay results obtained using the fluorescence procedure under validation to those from an established analytical procedure.
Validation criteria:
Precision
repeatability
Repeatability of the analytical procedure is assessed by measuring the concentrations of six independently prepared sample solutions at 100% of the assay test concentration. Alternatively, repeatability is assessed by measuring concentrations of three replicates of three separate sample solutions at different concentrations. The three concentrations should be sufficiently similar so that the repeatability is similar across the concentration range. If this is done, the repeatability at the three concentrations can be pooled for comparison to the acceptance criteria.
Validation criteria:
Intermediate Precision
The effect of random events on the analytical precision of the procedure should be evaluated. Typical variables include performing the analysis on different days, using different instrumentation, and having the method performed by two or more analysts. As a minimum, any combination of at least two of these factors totaling six experiments will provide an estimation of intermediate precision.
Validation criteria:
specificity
In fluorescence measurements, specificity is ensured by the use of a Reference Standard wherever possible and is demonstrated by the lack of interference from other components present in the matrix.
Validation criteria:
detection limit
Analysts can estimate the detection limit (DL) by calculating the standard deviation of NLT six replicate measurements of a blank solution and multiplying by 3.3. Alternatively, the standard deviation can be determined from the error of the intercept from a calibration curve or by demonstration that the signal-to-noise ratio is >3.3. Analysts must confirm the estimated DL by analyzing samples at the calculated concentration.
quantitation limit
Analysts can estimate the quantitation limit (QL) by calculating the standard deviation of NLT six replicate measurements of a blank solution and multiplying by 10. Alternatively, the standard deviation can be determined from the error at the intercept from a calibration curve or by demonstration that the signal-to-noise ratio is >10.
A sample solution prepared from a representative sample matrix spiked at the required QL concentration is measured to confirm sufficient sensitivity and adequate precision. The observed signal-to-noise ratio at the required QL should be >10.
Validation criteria:
linearity
A response curve between the analyte concentration and the fluorescence signal is prepared from NLT five Standard solutions at concentrations that encompass the anticipated concentration of the sample solution. Analysts then should evaluate the standard curve for linearity using appropriate statistical methods such as a least-squares regression. Deviation from linearity can result from either instrumental or sample factors, or both, and can be reduced to acceptable levels by reduction of the analyte concentration and thereby the associated absorbance values.
Validation criteria:
range
The operational range of an analytical instrument (and the analytical procedure as a whole) is the interval between the upper and lower concentrations (amounts) of analyte in the sample (including these concentrations) for which it has been demonstrated that the instrumental response function has a suitable level of precision, accuracy, and linearity.
Validation criteria:
robustness
Analysts should demonstrate the reliability of an analytical measurement by deliberate changes to experimental parameters. For fluorescence these changes can include measuring the stability of the analyte under specified storage conditions, varying pH, removal of oxygen, and adding possible interfering species, to list a few examples. Analysts should determine robustness concurrently using a suitable design-of-experiments procedure.
Verification
Analytical procedures described in USP–NF do not require validation. Instead, a verification is used to determine a procedure's suitability under actual conditions of use.
Thus the objective of fluorescence procedure verification is to demonstrate the suitability of a test procedure under actual conditions of use. Performance characteristics that verify the suitability of a fluorescence procedure are similar to those required for any analytical procedure. For additional information, see Verification of Compendial Procedures  1226
1226 for a discussion of the applicable general principles. Verification should be performed using a reference material and a well-defined matrix. Verification of compendial fluorescence procedures should at a minimum include the execution of the validation parameters for specificity, accuracy, precision, and quantitation limit, when appropriate, as indicated in Validation.
for a discussion of the applicable general principles. Verification should be performed using a reference material and a well-defined matrix. Verification of compendial fluorescence procedures should at a minimum include the execution of the validation parameters for specificity, accuracy, precision, and quantitation limit, when appropriate, as indicated in Validation.
Indirect Measurement Requirements
Some fluorescence procedures employ chromogenic reactions. Generally the requirements for the analytical performance characteristics should be used. In some instances the required accuracy and precision for the direct measurements may not be achievable. Under these circumstances, the accuracy and precision requirements may be widened by as much as 50%. However, any such widening must be justified on scientific grounds and with documented evidence. Under these circumstances, the amount of replication required to produce a scientifically sound reportable value may be increased. USP38
USP38
1
The rounded values are taken from ASTM Standard E388-04 (2009).
2
NIST SRM 2034 is no longer available.
3
The rounded values are taken from the intrinsic wavelength standard absorption band data from Travis et al. J Phys Chem Ref Data 2005;34(1):41. The maximum 95% measurement uncertainty is ±0.06 nm.
4
The red-shift value is taken from Parker CA. Raman spectra in spectrofluorimetry. Analyst. 1959;84:446–453.
5
Available from commercial vendors and from NIST as SRMs 2940 (orange emission), 2941 (green emission), 2942 (UV emission), 2943 (blue emission), and 2944 (red/NIR emission).
6
Commercial vendors provide a 1-mg/L solution of quinine sulfate dehydrate in 0.105 M perchloric acid that has been fully characterized by NIST as SRM 936a.
7
A series of day-to-day Intensity Standards is available from the German Federal Institute for Materials Research and Testing (BAM).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison (301) 816-8106 |
(GCCA2010) General Chapters - Chemical Analysis |
USP38–NF33 Page 652
Pharmacopeial Forum: Volume No. 40(1)



