INTRODUCTION
Electrical conductivity in water is a measure of the ion-facilitated electron flow through it. Water molecules dissociate into ions as a function of pH and temperature and result in a very predictable conductivity. Some gases, most notably carbon dioxide, readily dissolve in water and interact to form ions, which predictably affect conductivity also. For the purpose of this discussion, these ions and their resulting conductivity can be considered intrinsic to the water.
Water conductivity is also affected by the presence of extraneous ions. The extraneous ions used in modeling the conductivity specifications described below are the chloride and ammonia ions. The conductivity of the ubiquitous chloride ion (at the theoretical endpoint concentration of 0.47 ppm when chloride was a required attribute test in USP 22 and earlier revisions) and the ammonium ion (at the limit of 0.3 ppm) represents a major portion of the allowed water ionic impurity level. A balancing quantity of anions (such as chloride, to counter the ammonium ion) and cations (such as sodium, to counter the chloride ion) is included in this allowed impurity level to maintain electroneutrality. Extraneous ions such as these may have a significant effect on the water’s chemical purity and suitability for use in pharmaceutical applications.
The procedure in the section Bulk Water is specified for measuring the conductivity of waters such as Purified Water, Water for Injection, Water for Hemodialysis, and the condensate of Pure Steam. The procedure in the section Sterile Water is specified for measuring the conductivity of waters such as Sterile Purified Water, Sterile Water for Injection, Sterile Water for Inhalation, and Sterile Water for Irrigation.
The procedures below shall be performed using instrumentation that has been calibrated, has conductivity sensor cell constants that have been accurately determined, and has a temperature compensation function that has been disabled for Bulk Water Stage 1 testing. For both online and offline measurements, the suitability of instrumentation for quality control testing is also dependent on the sampling location(s) in the water system. The selected sampling instrument location(s) must reflect the quality of the water used.
INSTRUMENT SPECIFICATIONS AND OPERATING PARAMETERS
Water conductivity must be measured accurately with calibrated instrumentation. An electrical conductivity measurement consists of the determination of the conductance, G (or its inverse, resistance, R), of the fluid between and around the electrodes. The conductance (1/R) is directly affected by the geometrical properties of the electrodes; i.e., the conductance is inversely proportional to the distance (d) between the electrodes and proportional to the area (A) of the electrodes. This geometrical ratio (d/A) is known as the cell constant, 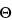 . Thus the measured conductance is normalized for the cell constant to determine the conductivity,
. Thus the measured conductance is normalized for the cell constant to determine the conductivity, 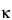 , according to the following equation:
, according to the following equation:
conductivity, 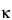 (S/cm) =
(S/cm) = 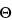 (cm–1)/R (W)
(cm–1)/R (W)
It is the cell constant and the resistance measurement that must be verified and adjusted, if necessary.
Cell constant:
The cell constant must be known within ±2%. The cell constant can be verified directly by using a solution of known or traceable conductivity, or indirectly by comparing the instrument reading taken with the conductivity sensor in question to readings from a conductivity sensor of known or traceable cell constant. If necessary, adjust the cell constant following the manufacturer¢s instrument protocol. The frequency of verification/calibration is a function of the sensor design.
Resistance measurement:
Calibration (or verification) of the resistance measurement is accomplished by replacing the conductivity sensor electrodes with precision resistors having standards traceable to NIST or equivalent national authorities in other countries (accurate to ±0.1% of the stated value) to give a predicted instrument conductivity response. The accuracy of the resistance measurement is acceptable if the measured conductivity with the traceable resistor is within ±0.1 µS/cm of the calculated value according to the equation above. For example, the traceable resistor is 50 kW, and the cell constant, 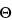 , is 0.10 cm–1. The calculated value is 2.0 × 10–6 S/cm or 2.0 µS/cm. The measured value should be 2.0 ± 0.1 µS/cm. The instrument must have a minimum resolution of 0.1 µS/cm on the lowest range.
System verification:
The cell constant of the user’s sensor can be determined with the user’s resistance measurement system, or the cell constant can be determined with an independent resistance measurement system. If the cell constant is determined with an independent resistance measurement system, it is recommended that the user verify that the sensor has been properly connected to the resistance measurement system to ensure proper performance. Verification can be made by comparing the conductivity (or resistivity) values displayed by the measuring equipment with those of an external calibrated conductivity-measuring device. The two non–temperature-compensated conductivity (or resistivity) values should be equivalent to or within ±5% of each other, or should have a difference that is acceptable on the basis of product water criticality and/or the water conductivity ranges in which the measurements are taken. The two conductivity sensors should be positioned close enough together to measure the same water sample at the same temperature and water quality.
, is 0.10 cm–1. The calculated value is 2.0 × 10–6 S/cm or 2.0 µS/cm. The measured value should be 2.0 ± 0.1 µS/cm. The instrument must have a minimum resolution of 0.1 µS/cm on the lowest range.
System verification:
The cell constant of the user’s sensor can be determined with the user’s resistance measurement system, or the cell constant can be determined with an independent resistance measurement system. If the cell constant is determined with an independent resistance measurement system, it is recommended that the user verify that the sensor has been properly connected to the resistance measurement system to ensure proper performance. Verification can be made by comparing the conductivity (or resistivity) values displayed by the measuring equipment with those of an external calibrated conductivity-measuring device. The two non–temperature-compensated conductivity (or resistivity) values should be equivalent to or within ±5% of each other, or should have a difference that is acceptable on the basis of product water criticality and/or the water conductivity ranges in which the measurements are taken. The two conductivity sensors should be positioned close enough together to measure the same water sample at the same temperature and water quality.
The target conductivity value(s) should be based on the type of water to be analyzed, and it should be equal to or less than the water conductivity limit for that type of water. Multiple measuring circuits may be embedded in the meter or the sensor, and each circuit may require separate verification or calibration before use. The frequency of recalibration is a function of instrument system design.
Temperature compensation and temperature measurements:
Because temperature has a substantial effect on conductivity readings of specimens at high and low temperatures, many instruments automatically correct the actual reading to display the value that theoretically would be observed at the nominal temperature of 25 . This is typically done using a temperature sensor embedded in the conductivity sensor and a software algorithm embedded in the instrument. This temperature compensation algorithm may not be accurate for the various water types and impurities. For this reason, conductivity values used in the Stage 1 test for Bulk Water are non–temperature-compensated measurements. Other conductivity tests that are specified for measurement at 25
. This is typically done using a temperature sensor embedded in the conductivity sensor and a software algorithm embedded in the instrument. This temperature compensation algorithm may not be accurate for the various water types and impurities. For this reason, conductivity values used in the Stage 1 test for Bulk Water are non–temperature-compensated measurements. Other conductivity tests that are specified for measurement at 25 can use either temperature-compensated or non–temperature-compensated measurements.
can use either temperature-compensated or non–temperature-compensated measurements.
A temperature measurement is required for the Stage 1 test or for the other tests at 25 . It may be made using the temperature sensor embedded in the conductivity cell sensor. An external temperature sensor positioned near the conductivity sensor is also acceptable. Accuracy of the temperature measurement must be ±2
. It may be made using the temperature sensor embedded in the conductivity cell sensor. An external temperature sensor positioned near the conductivity sensor is also acceptable. Accuracy of the temperature measurement must be ±2 .
.
BULK WATER
The procedure and test limits in this section are intended for Purified Water, Water for Injection, Water for Hemodialysis, the condensate of Pure Steam, and any other monographs that specify this section.
This is a three-stage test method to accommodate online or offline testing. Online conductivity testing provides real-time measurements and opportunities for real-time process control, decision, and intervention. Precautions should be taken while collecting water samples for offline conductivity measurements. The sample may be affected by the sampling method, the sampling container, and environmental factors such as ambient carbon dioxide concentration and organic vapors. This procedure can be started at Stage 2 if offline testing is preferred.
Procedure
stage 1
Stage 1 is intended for online measurement or may be performed offline in a suitable container.
1. Determine the temperature of the water and the conductivity of the water with a non–temperature-compensated conductivity reading.
2. Using Table 1, find the temperature value that is NMT the measured temperature, i.e., the next lower temperature. The corresponding conductivity value on this table is the limit. [Note—Do not interpolate. ]
3. If the measured conductivity is NMT the table value determined in step 2, the water meets the requirements of the test for conductivity. If the conductivity is higher than the table value, proceed with Stage 2.
Table 1. Stage 1—Temperature and Conductivity Requirements
(for non–temperature-compensated conductivity measurements only)
(for non–temperature-compensated conductivity measurements only)
| Temperature | Conductivity Requirement (µS/cm) |
|---|---|
| 0 | 0.6 |
| 5 | 0.8 |
| 10 | 0.9 |
| 15 | 1.0 |
| 20 | 1.1 |
| 25 | 1.3 |
| 30 | 1.4 |
| 35 | 1.5 |
| 40 | 1.7 |
| 45 | 1.8 |
| 50 | 1.9 |
| 55 | 2.1 |
| 60 | 2.2 |
| 65 | 2.4 |
| 70 | 2.5 |
| 75 | 2.7 |
| 80 | 2.7 |
| 85 | 2.7 |
| 90 | 2.7 |
| 95 | 2.9 |
| 100 | 3.1 |
stage 2
4. Transfer a sufficient amount of water to a suitable container, and stir the test specimen. Adjust the temperature, if necessary, and, while maintaining it at 25 ± 1 , begin vigorously agitating the test specimen while periodically observing the conductivity. When the change in conductivity (due to uptake of atmospheric carbon dioxide) is less than a net of 0.1 µS/cm per 5 min, note the conductivity. [Note—Conductivity measurements at this stage may be temperature-compensated to 25
, begin vigorously agitating the test specimen while periodically observing the conductivity. When the change in conductivity (due to uptake of atmospheric carbon dioxide) is less than a net of 0.1 µS/cm per 5 min, note the conductivity. [Note—Conductivity measurements at this stage may be temperature-compensated to 25 or non–temperature-compensated. ]
or non–temperature-compensated. ]
5. If the conductivity is not greater than 2.1 µS/cm, the water meets the requirements of the test for conductivity. If the conductivity is greater than 2.1 µS/cm, proceed with Stage 3.
stage 3
6. Perform this test within approximately 5 min of the conductivity determination in step 5, while maintaining the sample temperature at 25 ± 1 . Add a saturated potassium chloride solution to the same water sample (0.3 mL per 100 mL of the test specimen), and determine the pH to the nearest 0.1 pH unit, as directed in pH
. Add a saturated potassium chloride solution to the same water sample (0.3 mL per 100 mL of the test specimen), and determine the pH to the nearest 0.1 pH unit, as directed in pH  791
791 .
.
7. Referring to Table 2, determine the conductivity limit at the measured pH value. If the measured conductivity in step 4 is NMT the table value determined in step 6, the water meets the requirements of the test for conductivity. If either the measured conductivity is greater than this value or the pH is outside the range of 5.0–7.0, the water does not meet the requirements of the test for conductivity.
Table 2. Stage 3—pH and Conductivity Requirements
(for atmosphere- and temperature-equilibrated samples only)
(for atmosphere- and temperature-equilibrated samples only)
| pH | Conductivity Requirement (µS/cm) |
|---|---|
| 5.0 | 4.7 |
| 5.1 | 4.1 |
| 5.2 | 3.6 |
| 5.3 | 3.3 |
| 5.4 | 3.0 |
| 5.5 | 2.8 |
| 5.6 | 2.6 |
| 5.7 | 2.5 |
| 5.8 | 2.4 |
| 5.9 | 2.4 |
| 6.0 | 2.4 |
| 6.1 | 2.4 |
| 6.2 | 2.5 |
| 6.3 | 2.4 |
| 6.4 | 2.3 |
| 6.5 | 2.2 |
| 6.6 | 2.1 |
| 6.7 | 2.6 |
| 6.8 | 3.1 |
| 6.9 | 3.8 |
| 7.0 | 4.6 |
STERILE WATER
The procedure and test limits are intended for Sterile Purified Water, Sterile Water for Injection, Sterile Water for Inhalation, and Sterile Water for Irrigation, and any other monographs that specify this section. The sterile waters are derived from Purified Water or Water for Injection, and therefore have been determined to be compliant with the Bulk Water requirements before being stored in the container. The specification provided represents the maximum allowable conductivity value, taking into consideration the limitation of the measurement method and reasonable container leaching. Such specification and the sampling volume choices should be defined and validated on the basis of the intended purpose of the water.
Procedure
Obtain a sample that suitably reflects the quality of water used. Before opening, vigorously agitate the package to homogenize the water sample. Several packages may be required to collect sufficient water for analysis.
Transfer a sufficient amount of water to a suitable container, and stir the test specimen. Adjust the temperature, if necessary, and, while maintaining it at 25 ± 1 , begin vigorously agitating the test specimen while periodically observing the conductivity. When the change in conductivity (due to uptake of ambient carbon dioxide) is less than a net of 0.1 µS/cm per 5 min, note the conductivity.
, begin vigorously agitating the test specimen while periodically observing the conductivity. When the change in conductivity (due to uptake of ambient carbon dioxide) is less than a net of 0.1 µS/cm per 5 min, note the conductivity.
For containers with a nominal volume of 10 mL or less, if the conductivity is NMT 25 µS/cm, the water meets the requirements. For containers with a nominal volume greater than 10 mL, if the conductivity is NMT 5 µS/cm, the water meets the requirements.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| General Chapter | Antonio Hernandez-Cardoso, M.Sc.
Senior Scientific Liaison (301) 816-8308 |
(GCCA2010) General Chapters - Chemical Analysis |
USP38–NF33 Page 438
Pharmacopeial Forum: Volume No. 38(6)



