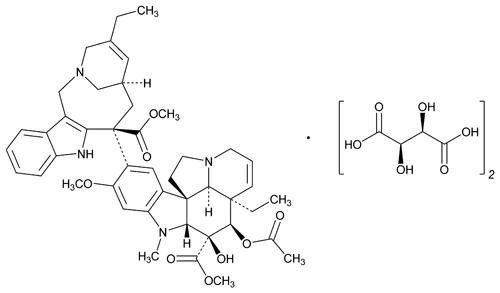
Vinorelbine Tartrate
C45H54N4O8·2C4H6O6 1079.11
 8¢-Norvincaleukoblastine,3¢,4¢-didehydro-4¢-deoxy-,[R-(R*,R*)]-2,3-dihydroxybutanedioate (1:2) (salt);
8¢-Norvincaleukoblastine,3¢,4¢-didehydro-4¢-deoxy-,[R-(R*,R*)]-2,3-dihydroxybutanedioate (1:2) (salt); USP35
USP35
3¢,4¢-Didehydro-4¢-deoxy-8¢-norvincaleukoblastine l-(+)-tartrate (1:2) (salt)

 [125317-39-7].
[125317-39-7].
 Assay.
Assay. USP35
USP35
(vin or' el been tar' trate).
Change to read:
C45H54N4O8·2C4H6O6 1079.11
3¢,4¢-Didehydro-4¢-deoxy-8¢-norvincaleukoblastine l-(+)-tartrate (1:2) (salt)
DEFINITION
Vinorelbine Tartrate contains NLT 98.0% and NMT 102.0% of C45H54N4O8·2C4H6O6, calculated on the anhydrous basis.
[Caution—Vinorelbine Tartrate is cytotoxic. Great care should be taken to prevent inhaling particles and exposing the skin to it.
]
IDENTIFICATION
• A. Infrared Absorption  197K
197K
Sample:
Dissolve 10 mg in 5 mL of water, add 0.5 mL of 5 N sodium hydroxide, and extract with 5 mL of methylene chloride. Filter the organic extract through anhydrous sodium sulfate, and evaporate the organic extract to a volume of about 0.5 mL.
Acceptance criteria:
Meets the requirements
Change to read:
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the
• C.
Sample solution:
Equivalent to 15 mg/mL of tartaric acid in water
Analysis:
To 0.1 mL of the Sample solution add 0.1 mL of 100 mg/mL of potassium bromide, 0.1 mL of 20 mg/mL of resorcinol, and 3 mL of sulfuric acid. Heat on a hot water bath for 5–10 min until a dark blue color develops. Allow to cool, and pour the solution into water.
Acceptance criteria:
The color changes to red (presence of tartrate).
ASSAY
Change to read:
• Procedure
Buffer:
Dissolve 6.9 g of monobasic sodium phosphate in 900 mL of water. Adjust with phosphoric acid to a pH of 4.2, and dilute with water to 1000 mL.
Mobile phase:
Dissolve 1.22 g of sodium 1-decanesulfonate in 620 mL of methanol, and add 380 mL of Buffer.
System suitability solution:
Prepare a solution containing 1.4 mg/mL of USP Vinorelbine Tartrate RS and 0.01 mg/mL of USP Vinorelbine Related Compound A RS in water. Expose a portion of this solution in a suitable xenon lamp apparatus capable of supplying a dose of 1600 KJ/m2 between 310 and 800 nm at a power of 500 W/m2 for 1 h to generate an additional photodegradation product,  3,6-epoxy vinorelbine.
3,6-epoxy vinorelbine. USP35
USP35
Standard solution:
1.4 mg/mL of USP Vinorelbine Tartrate RS in Mobile phase
Sample solution:
1.4 mg/mL of Vinorelbine Tartrate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 267 nm
Column:
3.9-mm × 15-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
 Resolution:
NLT 1.5
Resolution:
NLT 1.5 USP35 between vinorelbine and vinorelbine related compound A, System suitability solution
USP35 between vinorelbine and vinorelbine related compound A, System suitability solution
 Tailing factor:
NMT 2.0, Standard solution
Tailing factor:
NMT 2.0, Standard solution USP35
USP35
Relative standard deviation:
NLT 2.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of vinorelbine tartrate (C45H54N4O8·2C4H6O6 ) in the portion of Vinorelbine Tartrate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of vinorelbine from the Sample solution |
| rS | = | = peak response of vinorelbine from the Standard solution |
| CS | = | = concentration of USP Vinorelbine Tartrate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Vinorelbine Tartrate in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
Change to read:
• Organic Impurities
Buffer, Mobile phase, System suitability solution, Sample solution, and System suitability:
Proceed as directed in the Assay.
Standard stock solution:
Use the Standard solution as prepared in the Assay.
Standard solution:
0.28 µg/mL of vinorelbine tartrate in Mobile phase, from Standard stock solution
Chromatographic system:
Proceed as directed in the Assay, except use a run time of NLT three times the retention time of vinorelbine.
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Vinorelbine Tartrate taken:
Result = (rU/rT) × 100
| rU | = | = peak response for each impurity from the Sample solution |
| rT | = | = sum of the responses of all the peaks from the Sample solution |
Acceptance criteria:
See Table 1.
[Note—Disregard any peaks with an area less than or equal to one-half of the area of the peak for vinorelbine in the Standard solution. ]
Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|
| 3,6-Epoxy vinorelbinea | 0.8 | 0.3 | |
| Vinorelbine | 1.0 | — | |
| Vinorelbine related compound Ab | 1.2 | 0.2 | |
| Any unspecified impurityc | — | 0.2 | |
| Total Impuritiesd | — | 0.7 | |
|
a
b
4-O-Deacetylvinorelbine.
c
Any individual impurity or coeluted impurities comprising an individual peak.
d
Excluding 3,6-epoxy vinorelbine.
|
|||
SPECIFIC TESTS
• Clarity of Solution
Sample solution:
Equivalent to 10 mg/mL of anhydrous vinorelbine in water from Vinorelbine Tartrate
Acceptance criteria:
The solution is clear.
• Color of Solution
Sample solution:
Equivalent to 10 mg/mL of anhydrous vinorelbine in water from Vinorelbine Tartrate
Analysis:
Determine the absorbance of the Sample solution in a 1-cm cell at 420 nm in a suitable spectrophotometer, using water as the blank.
Acceptance criteria:
NMT 0.03
• pH  791
791 :
3.3–3.8, in a 10-mg/mL solution
:
3.3–3.8, in a 10-mg/mL solution
• Water Determination, Method Ia  921
921 :
NMT 4.0%
:
NMT 4.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers. Store in a freezer.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S.
Senior Scientific Liaison 1-301-816-8320 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 5027
Pharmacopeial Forum: Volume No. 37(1)