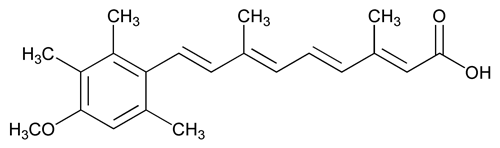
Acitretin
C21H26O3 326.43
2,4,6,8-Nonatetraenoic acid, 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-, (all-E)-;
(all-E)-9-(4-Methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoic acid

 [55079-83-9].
[55079-83-9].
(a'' si tre' tin).
C21H26O3 326.43
2,4,6,8-Nonatetraenoic acid, 9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-, (all-E)-;
(all-E)-9-(4-Methoxy-2,3,6-trimethylphenyl)-3,7-dimethyl-2,4,6,8-nonatetraenoic acid
DEFINITION
Acitretin contains NLT 98.0% and NMT 102.0% of C21H26O3, calculated on the dried basis.
[Caution—Acitretin is a teratogen. Great care should be taken when handling to avoid inhalation of dust or contact with skin.
]
[Note—Use low-actinic glassware and perform all tests under yellow and subdued light. ]
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
[Note—Store the solutions at 4 before injection. ]
before injection. ]
Mobile phase:
Alcohol, glacial acetic acid, and water (92:0.3:8)
System suitability stock solution:
0.01 mg/mL each of USP Acitretin RS and USP Tretinoin RS in alcohol. [Note—Dissolve in tetrahydrofuran before diluting with alcohol. ]
System suitability solution:
0.25 µg/mL each of USP Acitretin RS and USP Tretinoin RS in alcohol, from System suitability stock solution
Standard solution:
0.1 mg/mL of USP Acitretin RS in alcohol. [Note—Dissolve in tetrahydrofuran before diluting with alcohol. The final concentration of tetrahydrofuran in the preparation will be 2%. ]
Sample stock solution:
0.25 mg/mL of Acitretin in tetrahydrofuran and alcohol (1:19). [Note—Dissolve in tetrahydrofuran before diluting with alcohol. ]
Sample solution:
0.1 mg/mL of Acitretin in alcohol, from Sample stock solution
Chromatographic system
Mode:
LC
Detector:
UV 360 nm
Column:
4-mm × 25-cm; packing L1
Flow rate:
0.6 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for tretinoin and acitretin are 0.84 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 2.0 between tretinoin and acitretin, System suitability solution
Relative standard deviation:
NMT 1.0% of acitretin, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C21H26O3 in the portion of Acitretin taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Acitretin RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Acitretin in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the dried basis
IMPURITIES
Organic Impurities
[Note—Store the solutions at 4 before injection. ]
before injection. ]
• Procedure
Mobile phase and Chromatographic system:
Proceed as directed in the Assay.
Standard solution:
0.8 µg/mL each of USP Acitretin RS, USP Acitretin Related Compound A RS, and USP Acitretin Related Compound B RS in alcohol. [Note—Dissolve in tetrahydrofuran before diluting with alcohol. ]
Sample solution:
0.25 mg/mL of Acitretin in tetrahydrofuran and alcohol (1:19). [Note—Dissolve in tetrahydrofuran before diluting with alcohol. ]
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 1.5 between acitretin related compound A and acitretin; NLT 1.5 between acitretin related compound B and acitretin
Relative standard deviation:
NMT 10.0% for acitretin related compound A and NMT 10.0% for acitretin related compound B
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of acitretin related compound A and acitretin related compound B in the portion of Acitretin taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the relevant impurity from the Sample solution |
| rS | = | = peak response from the relevant impurity from the Standard solution |
| CS | = | = concentration of USP Acitretin Related Compound A RS or USP Acitretin Related Compound B RS in the Standard solution (µg/mL) |
| CU | = | = concentration of Acitretin in the Sample solution (µg/mL) |
Calculate the percentage of impurities other than acitretin related compounds A and B in the portion of Acitretin taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each individual unspecified impurity from the Sample solution |
| rS | = | = peak response of USP Acitretin RS in the Standard solution |
| CS | = | = concentration of USP Acitretin RS in the Standard solution (µg/mL) |
| CU | = | = concentration of Acitretin in the Sample solution (µg/mL) |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 1.0%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Acitretin related compound A | 0.78 | 0.3 |
| Acitretin | 1.0 | — |
| Acitretin related compound B | 1.61 | 0.3 |
| Any unspecified impurity | — | 0.1 |
| Total unspecified impurities | — | 0.4 |
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample in a vacuum at a pressure not exceeding 19 mm of mercury at 100
:
Dry a sample in a vacuum at a pressure not exceeding 19 mm of mercury at 100 for 4 h: it loses NMT 0.2% of its weight.
for 4 h: it loses NMT 0.2% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, protected from light. Store at controlled room temperature.
• USP Reference Standards  11
11
USP Acitretin Related Compound A RS
(2Z,4E,6E,8E)-9-(4-Methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoic acid.
C21H26O3 326.43
(2Z,4E,6E,8E)-9-(4-Methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoic acid.
C21H26O3 326.43
USP Acitretin Related Compound B RS
Ethyl (all-E)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoate.
C23H30O3 354.48
Ethyl (all-E)-9-(4-methoxy-2,3,6-trimethylphenyl)-3,7-dimethylnona-2,4,6,8-tetraenoate.
C23H30O3 354.48
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S.
Senior Scientific Liaison 1-301-816-8320 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2071
Pharmacopeial Forum: Volume No. 35(5) Page 1102