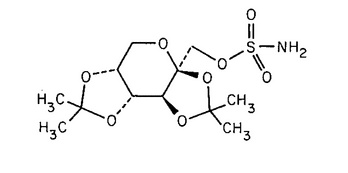
Topiramate
C12H21NO8S 339.36
 -d-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, sulfamate;
-d-Fructopyranose, 2,3:4,5-bis-O-(1-methylethylidene)-, sulfamate;
2,3:4,5-Di-O-isopropylidene- -d-fructopyranose sulfamate
-d-fructopyranose sulfamate


 [97240-79-4].
[97240-79-4].
(toe pir' a mate).
C12H21NO8S 339.36
2,3:4,5-Di-O-isopropylidene-
DEFINITION
Topiramate contains NLT 98.0% and NMT 102.0% of C12H21NO8S, calculated on the anhydrous basis.
[Caution—Great care must be exercised in handling Topiramate because it is a suspected teratogen.
]
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Mobile phase:
Acetonitrile and water (1:1)
Standard solution:
2 mg/mL of USP Topiramate RS in Mobile phase
Sample solution:
2 mg/mL of Topiramate in Mobile phase
Chromatographic system
(See Chromatography  621
621 .)
.)
Mode:
LC
Detector:
Refractive index
Column:
4.6-mm × 25-cm; 5-µm packing L1
Temperature
Column:
50
Detector
50
Flow rate:
0.6 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 1500 theoretical plates
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C12H21NO8S in the portion of Topiramate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Topiramate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Topiramate in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Organic Impurities
[Note—On the basis of the synthetic route, perform either Procedure 2 or Procedure 3. If N-methyltopiramate is a potential related compound, Procedure 1 and Procedure 3 are recommended. ]
• Procedure 1
Identification solution:
0.2 mg/mL of USP Topiramate Related Compound A RS in methanol
Standard solution A:
40 mg/mL of USP Topiramate RS in methanol
Standard solution B:
0.08 mg/mL of Topiramate from Standard solution A and methanol
Standard solution C:
0.04 mg/mL of Topiramate from Standard solution A and methanol
Sample solution:
40 mg/mL of Topiramate in methanol
Chromatographic system
Mode:
TLC
Adsorbent:
0.20-mm layer of chromatographic silica gel mixture, prewashed with methanol and air dried
Application volume:
20 µL
Developing solvent system:
Acetonitrile, methanol, and 0.5 M sodium chloride (7:3:10)
Spray reagent:
Prepare a 30-mg/mL solution of phenol in alcohol and concentrated sulphuric acid (95:5).
Analysis
Samples:
Standard solution B, Standard solution C, and Sample solution
Proceed as directed in the chapter. After elution, air-dry the plate, spray the plate with the Spray reagent, and let the plate air-dry. Then dry the plate for 10 min in an oven at 125 . [Note—The approximate RF values for topiramate and topiramate related compound A are 0.65 and 0.70, respectively. Disregard any spots at the origins of the chromatograms. Disregard any spot corresponding to topiramate related compound A because this impurity should be quantified using Procedure 2. ] Examine the plate using visible light, and estimate the percentage of all secondary spots in the chromatogram of the Sample solution by comparing each spot with the principal spot from the chromatograms of the Standard solutions.
. [Note—The approximate RF values for topiramate and topiramate related compound A are 0.65 and 0.70, respectively. Disregard any spots at the origins of the chromatograms. Disregard any spot corresponding to topiramate related compound A because this impurity should be quantified using Procedure 2. ] Examine the plate using visible light, and estimate the percentage of all secondary spots in the chromatogram of the Sample solution by comparing each spot with the principal spot from the chromatograms of the Standard solutions.
Acceptance criteria:
Any single spot is not greater in size and intensity than the spot for Standard solution C; NMT 0.1% of any individual impurity is found; and NMT 0.5% of total impurities by TLC is found.
• Procedure 2
Mobile phase:
Proceed as directed in the Assay.
[Note—Prepare all solutions fresh before use. ]
Sample solution:
40 mg/mL of Topiramate, in Mobile phase. [Note—Sonication may be used to aid dissolution. ]
System suitability solution:
0.3 mg/ml each of USP Fructose RS and USP Topiramate Related Compound A RS, in the Sample solution
Chromatographic system
Mode:
LC
Detector:
Refractive index detector
Column:
4.6-mm × 25-cm; 5-µm packing L1
Temperature
Column:
55
Detector:
55
Flow rate:
0.6 mL/min
Injection size:
50 µL
Run time:
NLT 5 times the retention time of the topiramate peak
System suitability
Sample:
System suitability solution
[Note—The relative retention times for fructose, topiramate related compound A, and topiramate are 0.45, 0.9, and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 1.0 between topiramate related compound A and topiramate
Relative standard deviation:
NMT 2.0% for the topiramate peak
Analysis
Sample:
Sample solution
Calculate the percentage of each of the impurities in the portion of Topiramate taken:
Result = (rU/rT) × (1/F) × 100
| rU | = | = peak area of any impurity |
| rT | = | = sum of the areas of all of the impurities peaks and the topiramate peak |
| F | = | = relative response factor, 1.2 for topiramate related compound A and 1.0 for all the other peaks |
Acceptance criteria:
See Impurity Table 1.
Total impurities:
NMT 0.5%
Impurity Table 1
| Name | Acceptance Criteria, NMT (%) |
|---|---|
| Fructose | 0.3 |
| Topiramate related compound A | 0.3 |
| Individual unknown | 0.1 |
• Procedure 3
Mobile phase:
Methanol and water (16:34)
Standard solution:
10 mg/mL of USP Topiramate RS and 0.04 mg/mL of USP Topiramate Related Compound A RS in Mobile phase
Sample solution:
10 mg/mL of Topiramate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
Refractive index
Column:
4.6-mm × 15-cm; 5-µm packing L15
Column temperature:
35 .
.
Flow rate:
1.5 mL/min
Injection size:
50 µL
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 1.0 between topiramate related compound A and topiramate
Relative standard deviation:
NMT 2.0% for the topiramate peak for six replicate injections
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each of the impurities in the portion of Topiramate taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak area of any impurity from the Sample solution |
| rS | = | = peak area of topiramate from the Standard solution |
| CS | = | = concentration of USP Topiramate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
| F | = | = relative response factor, 1.1 for topiramate related compound A and 1.0 for all the other peaks |
Acceptance criteria
Topiramate related compound A:
NMT 0.3%
Any other individual impurity:
NMT 0.10%
Total impurities detected by Procedure 3:
NMT 0.5%
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S :
:
 28.6
28.6 to
to  35.0
35.0 , measured at 20
, measured at 20
Sample solution:
4–10 mg/mL, in methanol
• Water Determination, Method I  921
921 :
NMT 0.5%
:
NMT 0.5%
• Limit of Sulfamate and Sulfate
[Note—Use water with resistivity NLT 18 megohm-cm for preparation of Mobile phase, Standard solution, and Sample solution. ]
Buffer:
0.8 g/L of p-hydroxybenzoic acid in water
Mobile phase:
Methanol and Buffer (2.5:97.5). Adjust with sodium hydroxide solution to a pH of 9.4 ± 0.5.
Standard solution:
4.5 µg/mL of sodium sulfate and 3.0 µg/mL of sulfamic acid in Mobile phase from anhydrous sodium sulfate and sulfamic acid, respectively
Sample solution:
6.0 mg/mL of topiramate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
Conductivity
Column:
4.6-mm × 15-cm; 5-µm packing L47
Detector temperature:
30
Flow rate:
1.5 mL/min
[Note—A suitable background suppression unit may be used. ]
Injection size:
70 µL
System suitability
Sample:
Standard solution
[Note—The relative retention time of the sulfamate peak is 0.44 relative to the sulfate peak. ]
Suitability requirements
Relative standard deviation:
NMT 15.0% for the sulfamate and sulfate peaks
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of sulfate ions in the portion of Topiramate taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response of sulfate ion from the Sample solution |
| rS | = | = peak response of sulfate ion from the Standard solution |
| CS | = | = concentration of sodium sulfate in the Standard solution (mg/mL) |
| CU | = | = concentration of Topiramate in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of the sulfate anion, 96.04 |
| Mr2 | = | = molecular weight of anhydrous sodium sulfate, 142.04 |
Calculate the percentage of sulfamate ions in the portion of Topiramate taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response of the sulfamate ion from the Sample solution |
| rS | = | = peak response of the sulfamate ion from the Standard solution |
| CS | = | = concentration of sulfamic acid in the Standard solution (mg/mL) |
| CU | = | = concentration of Topiramate in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of sulfamate anion, 96.09 |
| Mr2 | = | = molecular weight of sulfamic acid, 97.09 |
Acceptance criteria:
NMT 0.1% of sulfate; NMT 0.1% of sulfamate
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers, and store at controlled room temperature.
• Labeling:
If an Organic Impurities procedure other than Procedure 2 is used, then the labeling states the test with which the article complies. The label also states that it is a suspected teratogen.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4898
Pharmacopeial Forum: Volume No. 36(2) Page 433