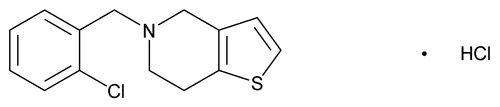
Ticlopidine Hydrochloride
C14H14ClNS·HCl 300.25
Thieno[3,2-c]pyridine, 5-[(2-chlorophenyl)methyl]-4,5,6,7-tetrahydro-, hydrochloride;
5-(o-Chlorobenzyl)-4,5,6,7-tetrahydrothieno-[3,2-c]pyridine hydrochloride

 [53885-35-1].
[53885-35-1].
(tye kloe' pi deen hye'' droe klor' ide).
C14H14ClNS·HCl 300.25
Thieno[3,2-c]pyridine, 5-[(2-chlorophenyl)methyl]-4,5,6,7-tetrahydro-, hydrochloride;
5-(o-Chlorobenzyl)-4,5,6,7-tetrahydrothieno-[3,2-c]pyridine hydrochloride
DEFINITION
Ticlopidine Hydrochloride contains NLT 98.0% and NMT 102.0% of C14H14ClNS·HCl, calculated on the dried basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• C. Identification Tests—General, Chloride  191
191 :
Meets the requirements
:
Meets the requirements
ASSAY
• Procedure
Buffer:
1.1 g of monobasic sodium phosphate and 0.28 g of dibasic sodium phosphate in 1000 mL of water. The pH of solution is between 6.1 and 6.6. If necessary, adjust to the required pH using phosphoric acid or sodium hydroxide.
Mobile phase:
Acetonitrile, methanol, and Buffer (6:7:7)
System suitability solution:
0.2 mg/mL of USP Ticlopidine Hydrochloride RS and 0.2 mg/mL of USP Sulconazole Nitrate RS in Mobile phase. [Note—Sonication may be necessary for complete dissolution. ]
Standard solution:
0.4 mg/mL of USP Ticlopidine Hydrochloride RS in Mobile phase
Sample solution:
0.4 mg/mL of Ticlopidine Hydrochloride in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 215 nm
Column:
4.6-mm × 25-cm; 10-µm packing L7
Flow rate:
2 mL/min
Column temperature:
40
Run time:
1.5 times the retention time of the ticlopidine peak
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 2.6 between ticlopidine hydrochloride and sulcanazole nitrate, System suitability solution
Relative standard deviation:
NMT 1.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C14H14ClNS·HCl in the portion of Ticlopidine Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of ticlopidine from the Sample solution |
| rS | = | = peak response of ticlopidine from the Standard solution |
| CS | = | = concentration of ticlopidine in the Standard solution |
| CU | = | = concentration of ticlopidine in the Sample solution |
IMPURITIES
Inorganic Impurities
• Residue on Ignition  231
231 :
NMT 0.1% on a 1-g sample
:
NMT 0.1% on a 1-g sample
• Heavy Metals, Method I  231
231 :
NMT 20 ppm
:
NMT 20 ppm
Organic Impurities
• Procedure 1
Adsorbent:
0.25-mm thickness of silica
Developing solvent:
Butanol, water, and glacial acetic acid (4:5:1). Shake well in a separatory funnel, allow it to settle, discard the lower aqueous layer, and use the upper organic layer.
Diluent:
Methylene chloride and methanol (1:2)
Iodine–methanol reagent:
Iodine TS and methanol (1:1)
Standard solution A:
15 mg/mL of USP Ticlopidine Hydrochloride RS in Diluent
Standard solution B:
2.5 mg/mL of each of USP Ticlopidine Related Compound A RS and USP Ticlopidine Related Compound B RS in Diluent
Sample solution:
15 mg/mL of Ticlopidine Hydrochloride in Diluent
Combined standard solution:
Transfer 1.5 mL of Standard solution B and 250 µL of the Sample solution to a 25-mL volumetric flask and dilute to volume with Diluent.
Application size:
2, 5, and 10 µL of the Combined standard solution and 20 µL of the Sample solution
Analysis
Samples:
Sample solution and Combined standard solution
Develop the plate to a distance of at least 15 cm from the origin, and remove the plate and air dry for at least 1 h. Analyze visually under UV light. Estimate the amounts of ticlopidine related compound A and ticlopidine related compound B. Spray the plate with the Iodine–methanol reagent, and estimate any other impurities by comparing to the ticlopidine hydrochloride spots in the Combined standard solution.
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Impurity Table 1
| Name | Retardation Factor (Rf) |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Ticlopidine hydrochloride | 1.00 | — |
| Ticlopidine hydrochloride related compound Aa | 1.26 | 0.5 |
| Ticlopidine hydrochloride related compound Bb | 1.41 | 0.5 |
|
a
(4-Oxo-4,5,6,7-tetrahydrothieno-[3,2-c]pyridine.
b
(5-(2-Chlorobenzyl)-4-oxo-4,5,6,7-tetrahydrothieno-[3,2-c]pyridine).
|
||
• Procedure 2
Buffer, Mobile phase, System suitability solution, Standard solution, Sample solution, and Chromatographic system:
Proceed as directed in the Assay.
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of N-methyl ticlopidine in the portion of Ticlopidine Hydrochloride taken:
Result = (rU/rT) × 100
| rU | = | = peak response of N-methyl ticlopidine in the Sample solution |
| rT | = | = sum of all the peak responses from in the Sample solution |
Calculate the percentage of any individual impurity in the portion of the Ticlopidine Hydrochloride taken:
Result = (rU/rS) × 100
| rU | = | = peak response of any impurity in the Sample solution |
| rS | = | = peak response of ticlopidine in the Standard solution |
Acceptance criteria
Individual impurities:
See Impurity Table 2.
Total impurities:
See Impurity Table 2.
Impurity Table 2
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Ticlopidine hydrochloride | 1 | — |
| N-Methyl ticlopidinea | 1.18 | 0.5 |
| Any other individual impurity | — | 0.10 |
| Total impuritiesb | — | 1.0 |
|
a
2-[N-Methyl-N-(2-chlorobenzyl)aminoethyl] thiophene hydrochloride.
b
Total of N-methyl ticlopidine and sum of % individual impurities from Procedure 1.
|
||
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a 1.0-g sample at 80
:
Dry a 1.0-g sample at 80 for 5 h: it loses NMT 1.0% of its weight.
for 5 h: it loses NMT 1.0% of its weight.
• Limit of Formaldehyde
Mobile phase:
Acetonitrile, water, and hydrochloric acid (3:2:0.004)
2,4 Dintirophenyl hydrazine solution:
1.65 mg/mL of 2,4-dinitrophenylhydrazine in acetonitrile
Standard stock solution:
Transfer a known amount of formaldehyde solution, equivalent to 37 mg of formaldehyde, into a 100-mL volumetric flask. Dilute with methanol to volume.
Standard solution:
Dilute the Standard stock solution with methanol to prepare a 1.85-µg/mL solution.
Sample solution:
0.50 g of Ticlopidine Hydrochloride in 10 mL methanol (sonication may be necessary for complete dissolution)
Derivatized standard and sample solutions:
Transfer 2.0 mL of 2,4-Dinitrophenyl hydrazine solution to five different 10-mL volumetric flasks: 50 µL of 2 N hydrochloric acid and 150, 250, and 500 µL of the Standard solution to the first three flasks; 500 µL of the Sample solution to the fourth; and 500 µL of methanol to the fifth flask. Mix each solution well and allow the solutions to react for at least 30 min at ambient temperature. Dilute each flask with Mobile phase to volume and mix well. The solutions should be analyzed within 4 h.
Chromatographic system
Mode:
LC
Detector:
UV 365 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Sample:
Derivatized standard solution prepared from 500 µL
Suitability requirements
Relative standard deviation:
NMT 10.0%
Analysis
Samples:
Derivatized standard solutions and Derivatized sample solution
[Note—The approximate retention time for 2,4 dinitrophenylhydrazine is about 3.5 min and for the formaldehyde and 2,4 dinitrophenylhydrazine derivative is about 3.8 min. ]
Calculate the formaldehyde concentration in ppm in the Derivatized sample solution as the concentration in the Ticlopidine Hydrochloride taken:
Result = (C × D)/W
| C | = | = concentration of formaldehyde from the calibration curve generated from the peak areas of the derivatized methanol and the three Derivatized standard solutions (µg/mL) |
| D | = | = dilution factor, 200 |
| W | = | = sample weight (g) |
Acceptance criteria
Formaldehyde:
NMT 20 ppm
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and store at a temperature below 30 .
.
• USP Reference Standards  11
11
USP Ticlopidine Hydrochloride Related Compound A RS
4-Oxo-4,5,6,7-tetrahydrothieno[3,2-c]pyridine.
4-Oxo-4,5,6,7-tetrahydrothieno[3,2-c]pyridine.
USP Ticlopidine Hydrochloride Related Compound B RS
5-(2-Chlorobenzyl)-4-oxo-4,5,6,7-tetrahydrothieno[3,2-c]pyridine.
5-(2-Chlorobenzyl)-4-oxo-4,5,6,7-tetrahydrothieno[3,2-c]pyridine.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Sujatha Ramakrishna, Ph.D.
Senior Scientific Liaison 1-301-816-8349 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4865
Pharmacopeial Forum: Volume No. 35(3) Page 582