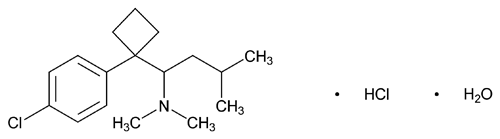
Sibutramine Hydrochloride
C17H29Cl2NO 334.32
Cyclobutanemethanamine, 1-(4-chlorophenyl)-N,N-dimethyl- -(2-methylpropyl)-, hydrochloride, monohydrate, (±)-;
-(2-methylpropyl)-, hydrochloride, monohydrate, (±)-;
(±)-1-(p-Chlorophenyl)- -isobutyl-N,N-dimethylcyclobutanemethylamine hydrochloride monohydrate
-isobutyl-N,N-dimethylcyclobutanemethylamine hydrochloride monohydrate


 [125494-59-9].
[125494-59-9].
(si bue' tra meen hye'' droe klor' ide).
C17H29Cl2NO 334.32
Cyclobutanemethanamine, 1-(4-chlorophenyl)-N,N-dimethyl-
(±)-1-(p-Chlorophenyl)-
DEFINITION
Sibutramine Hydrochloride contains NLT 98.0% and NMT 102.0% of C17H29Cl2NO, calculated on the anhydrous basis.
IDENTIFICATION
• B.
It meets the requirements of the tests for Chloride  191
191 .
.
Sample solution:
1 mg/mL of Sibutramine Hydrochloride in 3 M nitric acid
• C. Optical Rotation, Specific Rotation  781
781 :
Between
:
Between  0.1
0.1 and +0.1
and +0.1 , determined at 20
, determined at 20
Sample solution:
Transfer 3.0 g of Sibutramine Hydrochloride into a 20-mL volumetric flask, and dissolve in 15 mL of alcohol. Place in a water bath at 20 , and after about 15 min dilute with alcohol to volume, and mix.
, and after about 15 min dilute with alcohol to volume, and mix.
ASSAY
• Procedure
Mobile phase:
Dissolve 1.0 g of 1-butanesulfonic acid sodium salt monohydrate in 675 mL of water, and adjust with phosphoric acid to a pH of 3.0. Add 325 mL of acetonitrile and 10 mL of tetrahydrofuran, and mix.
Internal standard solution:
Prepare a 0.75% w/v solution of 4-bromophenol in methanol, and mix.
Standard stock solution:
Transfer a known quantity of USP Sibutramine Hydrochloride RS into a suitable volumetric flask, add the Internal standard solution equivalent to 10% of the final volume, and dilute with methanol to volume to obtain a solution containing a known concentration of 1.0 mg/mL of Sibutramine Hydrochloride.
Standard solution:
Dilute the Standard stock solution in methanol (1:10), and mix.
System suitability solution:
0.5 mg/mL of USP Sibutramine Related Compound D RS in methanol. Pipette 1.0 mL of this solution into a 50-mL volumetric flask, add 50 mg of USP Sibutramine Hydrochloride RS, dissolve in and dilute with methanol to volume, and mix. Transfer 10.0 mL of this solution into a 100-mL volumetric flask, add 1.0 mL of Internal standard solution and 90 mL of methanol, and mix.
Sample stock solution:
Transfer 100 mg of Sibutramine Hydrochloride into a 100-mL volumetric flask, add 10.0 mL of Internal standard solution, and dilute with methanol to volume.
Sample solution:
Dilute the Sample stock solution in methanol (1:10), and mix.
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
3.9-mm × 30-cm; packing L1
Column temperature:
30
Flow rate:
2 mL/min
Injection size:
15 µL
System suitability
Samples:
Standard solution, System suitability solution, and Sample solution
Suitability requirements
Resolution:
NLT 1.4 between sibutramine and sibutramine related compound D, System suitability solution
Peak ratio agreement:
The duplicates of the ratio for the peak areas of sibutramine to the internal standard agree to within 1.0%, Standard solution and Sample solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C17H29Cl2NO in the portion of Sibutramine Hydrochloride taken:
Result = (RU/RS) × (CS/CU) × 100
| RU | = | = peak area ratios of sibutramine to the internal standard from the Sample solution |
| RS | = | = peak area ratios of sibutramine to the internal standard from the Standard solution |
| CS | = | = concentration of USP Sibutramine Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of Sibutramine Hydrochloride in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%, determined on 1.5 g. Ignite at 450
:
NMT 0.1%, determined on 1.5 g. Ignite at 450 . [Note—Use the residue obtained for the Heavy Metals test. ]
. [Note—Use the residue obtained for the Heavy Metals test. ]
• Heavy Metals
Phenolphthalein solution:
1.0% w/v solution of phenolphthalein in 50% alcohol solution
Acetate buffer pH 3.5:
Dissolve 25 g of ammonium acetate in 25 mL of water, and add 27.5 mL of concentrated hydrochloric acid. Measure the pH, and adjust if necessary, with either 2 M hydrochloric acid or 3 M ammonia solution to a pH of 3.5. Dilute with water to 100 mL, and mix.
Standard lead solution:
Dissolve 0.16 g of lead nitrate in 80 mL of water containing 1 mL of concentrated nitric acid. Dilute with water to 1000 mL. Dilute 10 mL of this solution with water to 50 mL. Dilute 5 mL of this solution with water to 100 mL. This solution contains 1 µg of lead per mL.
Sample solution:
Determine heavy metals from the residue retained from the test for Residue on Ignition. Dissolve the residue in 0.5 mL of concentrated hydrochloric acid. Evaporate the solution on a water bath to dryness. Dissolve the residue in 2 mL of water. Add about 3 drops of the Phenolphthalein solution, and neutralize with 1 M sodium hydroxide solution. Dilute with water to 15 mL, and mix.
Analysis:
Transfer 12.0 mL of the Sample solution to a Nessler cyclinder. Place 2 mL of the Sample solution into a second Nessler cylinder, and add 10 mL of the Standard lead solution. Treat the two Nessler cylinders equally. Add 1.2 mL of thioacetamide–glycerin base TS, and mix. Then add 2 mL of Acetate buffer pH 3.5, and mix. Allow the solutions to stand for 2 min. The Sample solution is not more intensely colored than the Standard lead solution.
Acceptance criteria:
NMT 10 ppm of heavy metals (as lead) is found.
Organic Impurities
• Procedure
Diluent:
Methanol, dichloromethane, and 1 M sodium hydroxide (10:10:1)
System suitability stock solution:
0.1 mg/mL each of USP Sibutramine Related Compound A RS, USP Sibutramine Related Compound B RS, USP Sibutramine Related Compound C RS, and USP Sibutramine Related Compound D RS in Diluent. Dilute this solution in Diluent (1:10), and mix.
System suitability solution:
10 mg/mL of USP Sibutramine Hydrochloride RS in System suitability stock solution
Sample solution:
10 mg/mL of Sibutramine Hydrochloride previously ground in Diluent
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.5-mm × 15-m; coated with 0.5-µm phase G5
Temperature
Injector:
200
Detector:
250
Column:
See the temperature program table below.
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 100 | 0 | 100 | 10 |
| 100 | 1 | 130 | 0 |
| 130 | 5 | 175 | 10 |
| 175 | 1.5 | 215 | 15 |
Carrier gas:
Nitrogen
Flow rate:
7.5 mL/min. [Note—The flow rate is adjusted so that the main peak is eluted after about 20 min. ]
Injection size:
1.0 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 1.3 between sibutramine related compound A and sibutramine related compound B
Analysis
Sample:
Sample solution
Calculate the percentage of each impurity in the portion of Sibutramine Hydrochloride taken:
Result = (rU/rT) × 100
| rU | = | = response of each individual impurity from the Sample solution |
| rT | = | = sum of the responses of all peaks from the Sample solution |
Acceptance criteria
Individual impurities:
See Impurity Table 1. [Note—Disregard any unspecified impurity peaks less than 0.05%. ]
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| N-{3-Methyl-1-(1-phenylcyclobutyl)butyl}-N,N-dimethylamine hydrochloride | 0.33 | 0.1 |
| N-[1-(4-Chlorophenyl) cyclobutylmethyl]-N,N-dimethylamine hydrochloride | 0.42 | 0.1 |
| Sibutramine related compound Aa | 0.73 | 0.1 |
| Sibutramine related compound Bb | 0.83 | 0.1 |
| Sibutramine related compound Cc | 1.14 | 0.1 |
| Sibutramine related compound Dd | 1.19 | 0.1 |
| 1-[1-(4-Chlorophenyl)cyclobutyl]-3-methylbutylamine hydrochloride | 1.45 | 0.1 |
| (N-{1-[1-(4-Chlorophenyl)cyclobutyl]-2-phenylethyl}-N-N-dimethylamine hydrochloride) | 2.2 | 0.1 |
| Total specified impurities | — | 0.3 |
| Any unspecified impurities | — | 0.1 |
| Total unspecified impurities | — | 0.2 |
|
a
N-{1-[1-(2-Chlorophenyl)cyclobutyl]-3-methylbutyl}-N,N-dimethylamine hydrochloride.
b
N-{1-[1-(3-Chlorophenyl)cyclobutyl]-3-methylbutyl}-N,N-dimethylamine hydrochloride.
c
N-{1-[1-(4-Chlorophenyl)cyclobutyl]pentyl}-N,N-dimethylamine hydrochloride.
d
N-{1-[1-(4-Chlorophenyl)cyclobutyl]-3-methylbutyl}-N-methylamine hydrochloride.
|
||
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
Between 4.5% and 6.0%
:
Between 4.5% and 6.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers. Store at room temperature.
• USP Reference Standards  11
11
USP Sibutramine Related Compound A RS
N-{1-[1-(2-Chlorophenyl)cyclobutyl]-3-methylbutyl}-N,N-dimethylamine hydrochloride.
N-{1-[1-(2-Chlorophenyl)cyclobutyl]-3-methylbutyl}-N,N-dimethylamine hydrochloride.
USP Sibutramine Related Compound B RS
N-{1-[1-(3-Chlorophenyl)cyclobutyl]-3-methylbutyl}-N,N-dimethylamine hydrochloride.
N-{1-[1-(3-Chlorophenyl)cyclobutyl]-3-methylbutyl}-N,N-dimethylamine hydrochloride.
USP Sibutramine Related Compound C RS
N-{1-[1-(4-Chlorophenyl)cyclobutyl]pentyl}-N,N-dimethylamine hydrochloride.
N-{1-[1-(4-Chlorophenyl)cyclobutyl]pentyl}-N,N-dimethylamine hydrochloride.
USP Sibutramine Related Compound D RS
N-{1-[1-(4-Chlorophenyl)cyclobutyl]-3-methylbutyl}-N-methylamine hydrochloride.
N-{1-[1-(4-Chlorophenyl)cyclobutyl]-3-methylbutyl}-N-methylamine hydrochloride.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4634
Pharmacopeial Forum: Volume No. 34(4) Page 986