Add the following:
(roe'' si gli' ta zone mal' ee ate).
C18H19N3O3S·C4H4O4 473.50
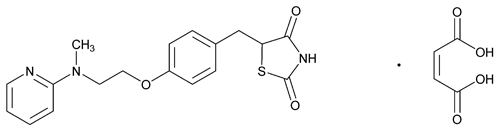
(±)-5-[p-[2-(Methyl-2-pyridylamino)ethoxy]benzyl]-2,4-thiazolidinedione maleate (1:1);
(RS)-5-{[4-({2-[Methyl(2-pyridinyl)amino]ethyl}oxy)phenyl]methyl}-1,3-thiazolidine-2,4-dione (Z)-2-butenedioate
DEFINITION
Rosiglitazone Maleate contains NLT 98.0% and NMT 102.0% of C18H19N3O3S·C4H4O4, calculated on the anhydrous and solvent-free basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Buffer:
Dissolve 5.75 g of phosphoric acid in 800 mL water, adjust with 4 N sodium hydroxide to a pH of 3.0, and dilute with water to 1 L.
Mobile phase:
Acetonitrile and Buffer (25:75)
System suitability solution:
Transfer 2.5 mg of USP Rosiglitazone Maleate RS and 1 mg of USP Rosiglitazone Related Compound A RS to a 50-mL volumetric flask, dissolve in 1 mL of stabilizer-free tetrahydofuran, and dilute with Mobile phase to volume.
Standard solution:
0.05 mg/mL of USP Rosiglitazone Maleate RS in Mobile phase
Sample solution:
0.05 mg/mL of Rosiglitazone Maleate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 235 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
Greater than 2.0 between rosiglitazone and rosiglitazone related compound A, System suitability solution
Tailing factor:
NMT 2.0, Standard solution
Relative standard deviation:
NMT 1.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of rosiglitazone maleate (C18H19N3O3S·C4H4O4) in the portion of Rosiglitazone Maleate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Rosiglitazone Maleate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Rosiglitazone Maleate in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous and solvent-free basis
OTHER COMPONENTS
• Content of Maleic Acid
Buffer:
Prepare 0.1 M sodium phosphate buffer as follows. Add 11.5 g of phosphoric acid to 800 mL of water, adjust with 2 N sodium hydroxide to a pH of 3.0, and dilute with water to 1 L.
Mobile phase:
Methanol and Buffer (50:50)
Diluent:
Methanol and water (50:50)
System suitability solution:
0.1 µg/mL of USP Fumaric Acid RS and 0.04 mg/mL of USP Rosiglitazone Maleate RS in Diluent
Standard solution:
0.01 mg/mL of USP Maleic Acid RS in Diluent
Sample solution:
0.04 mg/mL of Rosiglitazone Maleate in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 212 nm
Column:
4.6-mm × 15-cm; 5-µm packing L14
Column temperature:
40
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for rosiglitazone, maleic acid, and fumaric acid are 0.5, 1.0, and 1.8, respectively. ]
Suitability requirements
Resolution:
NLT 2.0 between maleic acid and fumaric acid, System suitability solution
Relative standard deviation:
NMT 2.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of maleic acid in the portion of Rosiglitazone Maleate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of maleic acid from the Sample solution |
| rS | = | = peak response of maleic acid from the Standard solution |
| CS | = | = concentration of USP Maleic Acid RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Rosiglitazone Maleate in the Sample solution (mg/mL) |
Acceptance criteria:
23.5%–26.0%
IMPURITIES
• Residue on Ignition,  281
281 :
NMT 0.2%, using an ignition temperature of 800 ± 25
:
NMT 0.2%, using an ignition temperature of 800 ± 25
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
• Organic Impurities
[Note—Protect the System suitability solution and Sample solution from light. ]
Buffer 1:
Prepare 0.05 M dibasic potassium phosphate buffer as follows. Dissolve 11.4 g of dibasic potassium phosphate trihydrate in 800 mL of water, adjust with a mixture of phosphoric acid and water (1:1) to a pH of 7.0, and dilute with water to 1 L.
Solution A:
Acetonitrile and Buffer 1 (30:70)
Solution B:
Acetonitrile and Buffer 1 (70:30)
Mobile phase:
See Table 1. Return to original conditions and re-equilibrate the system.
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 25.0 | 100 | 0 |
| 50.0 | 0 | 100 |
Buffer 2:
Prepare 0.05 M monobasic potassium phosphate buffer by dissolving 6.8 g of monobasic potassium phosphate in 1 L of water.
Diluent:
Acetonitrile and Buffer 2 (30:70)
System suitability solution:
0.5 mg/mL of USP Rosiglitazone Maleate RS in Diluent, using sonication, if necessary, to dissolve. [Note—USP Rosiglitazone Maleate RS contains rosiglitazone related compound A as a minor component. ]
Sample solution:
0.5 mg/mL of Rosiglitazone Maleate in Diluent, using sonication, if necessary, to dissolve
Chromatographic system
Mode:
LC
Detector:
UV 246 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
[Note—Identify the peak due to rosiglitazone related compound A based on its relative retention time shown in Table 2. ]
Suitability requirements
Resolution:
NLT 2.0 between rosiglitazone and rosiglitazone related compound A
Analysis
Sample:
Sample solution
Calculate the percentage of any individual impurity in the portion of Rosiglitazone Maleate taken:
Result = (rU/rT) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rT | = | = sum of the peak responses from the Sample solution, except for the peak response of maleic acid |
Acceptance criteria:
See Table 2.
Table 2
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Maleic acid | 0.09 | Disregard |
| Impurity 1a | 0.15 | 0.1 |
| Impurity 2b | 0.81 | 0.5 |
| Rosiglitazone | 1.0 | — |
| Rosiglitazone related compound Ac | 1.15 | 0.5 |
| Any other individual impurity | — | 0.1 |
| Total impurities | — | 1.0 |
|
a
2-(5-{[4-({2-[Methyl(2-pyridinyl)amino]ethyl}oxy)phenyl]methyl}-2,4-dioxo-1,3-thiazolidin-3-yl)butanedioic acid.
b
3-[4-({2-[Methyl(2-pyridinyl)amino]ethyl}oxy)phenyl]propanamide.
c
(5Z)-5-{[4-({2-[Methyl(2-pyridinyl)amino]ethyl}oxy)phenyl]methylidene}-1,3-thiazolidine-2,4-dione.
|
||
SPECIFIC TESTS
• Water Determination, Method 1a  921
921 :
NMT 0.5%
:
NMT 0.5%
[Note—Because maleic acid will react with methanol thereby producing water, both titrant and solvent must be methanol free. ]
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and store at room temperature.
• USP Reference Standards  11
11
USP Rosiglitazone Related Compound A RS
(5Z)-5-{[4-({2-[Methyl(2-pyridinyl)amino]ethyl}oxy) phenyl]methylidene}-1,3-thiazolidine-2,4-dione.
C18H17N3O3S 355.41
(5Z)-5-{[4-({2-[Methyl(2-pyridinyl)amino]ethyl}oxy) phenyl]methylidene}-1,3-thiazolidine-2,4-dione.
C18H17N3O3S 355.41
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4592
Pharmacopeial Forum: Volume No. 37(1)