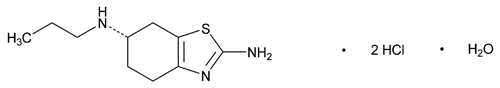
Pramipexole Dihydrochloride
C10H17N3S·2HCl·H2O 302.26
Benzothiazole-2,6-diamine, 4,5,6,7-tetrahydro-N6-propyl-, dihydrochloride, monohydrate, (S)-;
(S)-2-Amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate

 [191217-81-9].
[191217-81-9].
(pram'' i pex' ole dye hye'' droe klor' ide).
C10H17N3S·2HCl·H2O 302.26
Benzothiazole-2,6-diamine, 4,5,6,7-tetrahydro-N6-propyl-, dihydrochloride, monohydrate, (S)-;
(S)-2-Amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole dihydrochloride monohydrate
DEFINITION
Pramipexole Dihydrochloride contains NLT 98.0% and NMT 102.0% of C10H19Cl2N3S, calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption  197A
197A or
or  197M
197M
• B.
The retention time of the major peak in the Sample solution corresponds to that of pramipexole (S-enantiomer) in the System suitability solution in the test for Enantiomeric Purity.
• C. Identification Tests—General, Chloride  191
191
Sample:
1 mg/mL of Pramipexole Dihydrochloride in water
Acceptance criteria:
Meets the requirements of the silver nitrate precipitate test
ASSAY
• Procedure
Solution A:
Dissolve 9.1 g of potassium dihydrogen phosphate and 5.0 g of sodium 1-octanesulfonate monohydrate in 1 L of water. Adjust with phosphoric acid to a pH of 3.0.
Solution B:
Acetonitrile and Solution A (1:1)
Diluent:
Acetonitrile and Solution A (1:4)
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A
(%) |
Solution B
(%) |
|---|---|---|
| 0 | 60 | 40 |
| 15 | 20 | 80 |
| 15.1 | 60 | 40 |
| 20 | 60 | 40 |
System suitability solution:
1.5 mg/mL of USP Pramipexole Dihydrochloride RS and 0.8 mg/mL of USP Pramipexole Related Compound A RS in Diluent
Standard solution:
1.5 mg/mL of USP Pramipexole Dihydrochloride RS in Diluent
Sample solution:
1.5 mg/mL of Pramipexole Dihydrochloride in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 264 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
40 ± 5
Flow rate:
1.5 mL/min
Injection size:
5 µL
System suitability
Samples:
System suitability solution and Standard solution [Note—The relative retention times for pramipexole related compound A and pramipexole are about 0.7 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 6.0 between pramipexole related compound A and pramipexole, System suitability solution
Tailing factor:
NMT 2.0 for pramipexole, System suitability solution
Relative standard deviation:
NMT 1.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C10H19Cl2N3S in the portion of Pramipexole Dihydrochloride taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Pramipexole Dihydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of pramipexole dihydrochloride, 284.26 |
| Mr2 | = | = molecular weight of pramipexole dihydrochloride monohydrate, 302.26 |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.10%
:
NMT 0.10%
• Heavy Metals, Method I  231
231
Standard solution:
Standard Lead Solution, 10 ppm
Sample solution:
Ash 2 g of Pramipexole Dihydrochloride until an almost dry, carbonized mass is obtained. Cool the residue, add 2.0 mL of concentrated nitric acid and 5 drops of concentrated sulfuric acid, and carefully allow the fumes to evolve. Ignite at 500 –600
–600 until the carbon is completely burned off. Cool the residue, add 4 mL of 6 M hydrochloric acid, cover the crucible, and digest on a boiling water bath for 15 min. Evaporate to dryness. Add one drop of concentrated hydrochloric acid and 10 mL of hot water, and digest for a further 2 min on the boiling water bath. Add 6 M ammonia solution dropwise until the solution is weakly alkaline, and adjust with 1 M acetic acid to a pH of 3.0–4.0. Filter the solution into a 25-mL volumetric flask, and dilute with water to 25 mL by washing the crucible and the filter.
until the carbon is completely burned off. Cool the residue, add 4 mL of 6 M hydrochloric acid, cover the crucible, and digest on a boiling water bath for 15 min. Evaporate to dryness. Add one drop of concentrated hydrochloric acid and 10 mL of hot water, and digest for a further 2 min on the boiling water bath. Add 6 M ammonia solution dropwise until the solution is weakly alkaline, and adjust with 1 M acetic acid to a pH of 3.0–4.0. Filter the solution into a 25-mL volumetric flask, and dilute with water to 25 mL by washing the crucible and the filter.
Acceptance criteria:
NMT 10 ppm
Organic Impurities
• Procedure
Solution A, Solution B, Diluent, Mobile phase, and Chromatographic system:
Proceed as directed in the Assay.
System suitability solution:
7.5 µg/mL of USP Pramipexole Dihydrochloride RS and 3 µg/mL of USP Pramipexole Related Compound A RS in Diluent
Standard solution:
1.5 µg/mL of USP Pramipexole Dihydrochloride RS in Diluent
Sample solution:
1.5 mg/mL of Pramipexole Dihydrochloride in Diluent
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 6.0 between pramipexole related compound A and pramipexole, System suitability solution
Tailing factor:
NMT 2.0 for pramipexole, System suitability solution
Relative standard deviation:
NMT 5.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of any individual impurity in the portion of Pramipexole Dihydrochloride taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of pramipexole from the Standard solution |
| CS | = | = concentration of USP Pramipexole Dihydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of pramipexole dihydrochloride monohydrate in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of pramipexole dihydrochloride, 284.26 |
| Mr2 | = | = molecular weight of pramipexole dihydrochloride monohydrate, 302.26 |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.5%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|
| Pramipexole propionamidea | 0.5 | 0.15 | |
| Pramipexole related compound Ab | 0.7 | 0.15 | |
| Pramipexole | 1.0 | — | |
| N-Propylpramipexolec | 1.4 | 0.15 | |
| Pramipexole dimerd | 1.7 | 0.15 | |
| Any other unidentified individual impurity | — | 0.10 | |
|
a
(S)-N-(2-Amino-4,5,6,7-tetrahydrobenzothiazol-6-yl)propionamide.
b
(S)-4,5,6,7-Tetrahydrobenzothiazole-2,6-diamine.
c
(S)-2,6-Dipropylamino-4,5,6,7-tetrahydrobenzothiazole.
d
N6,N6'-[2-Methylpentane-1,3-diyl]bis(4,5,6,7-tetrahydrobenzothiazole-2,6-diamine). This is a dimer of pramipexole (a mixture of four possible isomers).
|
|||
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
NLT 4.5% and NMT 6.5%
:
NLT 4.5% and NMT 6.5%
• Enantiomeric Purity
Mobile phase:
n-Hexane, dehydrated alcohol, and diethylamine (850:150:1)
System suitability stock solution:
1 mg/mL each of USP Pramipexole Dihydrochloride RS and USP Pramipexole Related Compound D RS in dehydrated alcohol
System suitability solution:
0.01 mg/mL each of USP Pramipexole Dihydrochloride RS and USP Pramipexole Related Compound D RS from System suitability stock solution in Mobile phase
Standard stock solution:
2.0 mg/mL of USP Pramipexole Related Compound D RS in dehydrated alcohol
Standard solution:
1.5 µg/mL of USP Pramipexole Related Compound D RS in Mobile phase
Sample solution:
0.3 mg/mL, prepared by dissolving a suitable weighed quantity of Pramipexole Dihydrochloride in 25% of a flask volume of dehydrated alcohol and diluting with Mobile phase to volume
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 25-cm; 10-µm packing L51
Flow rate:
1.5 mL/min
Sample size:
75 µL
System suitability
Samples:
System suitability solution [Note—The relative retention times for pramipexole related compound D (R-enantiomer) and pramipexole (S-enantiomer) are 0.5 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 5.0 between pramipexole related compound D and pramipexole, System suitability solution
Tailing factor:
NMT 2.4 for pramipexole, System suitability solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of pramipexole related compound D in the portion of Pramipexole Dihydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of pramipexole related compound D from the Sample solution |
| rS | = | = peak response of pramipexole related compound D from the Standard solution |
| CS | = | = concentration of pramipexole related compound D in the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
Acceptance criteria:
NMT 1.0% of pramipexole related compound D
• Limit of Palladium
[Note—Perform this test if palladium is a known inorganic impurity. ]
Diluent:
0.1 M hydrochloric acid
Standard solution:
40 µg/L of palladium in Diluent, from commercially available palladium standard solution for atomic absorption/inductively coupled plasma. [Note—Freshly prepare this solution as required on the day of use. ]
Sample solution:
To 0.5 g of Pramipexole Dihydrochloride in a 50-mL volumetric flask add 5.00 mL of 1 M hydrochloric acid, and dissolve with heating. Cool to room temperature, and dilute with water to volume.
Spectrometric conditions
Mode:
Atomic absorption spectrophotometry
Analytical wavelength:
Palladium emission line at 247.6 nm
Lamp:
Hollow cathode
Atomization source:
Graphite furnace. [Note—Follow the manufacturer’s recommended programming sequence. ]
Sample size:
20 µL
Blank:
Diluent
System suitability
Sample:
Standard solution
Suitability requirements
Absorbance:
NLT 0.034
Analysis
Samples:
Standard solution and Sample solution
Determine the concentration of palladium in the Sample solution by the standard addition method.
Acceptance criteria:
NMT 5 ppm
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers, protected from moisture and light.
• USP Reference Standards  11
11
USP Pramipexole Related Compound A RS
(S)-4,5,6,7-Tetrahydrobenzothiazole-2,6-diamine.
C7H11N3S 169.25
(S)-4,5,6,7-Tetrahydrobenzothiazole-2,6-diamine.
C7H11N3S 169.25
USP Pramipexole Related Compound D RS
(R)-2-Amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole.
C10H17N3S 211.33
(R)-2-Amino-4,5,6,7-tetrahydro-6-(propylamino)benzothiazole.
C10H17N3S 211.33
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4384
Pharmacopeial Forum: Volume No. 36(3) Page 676