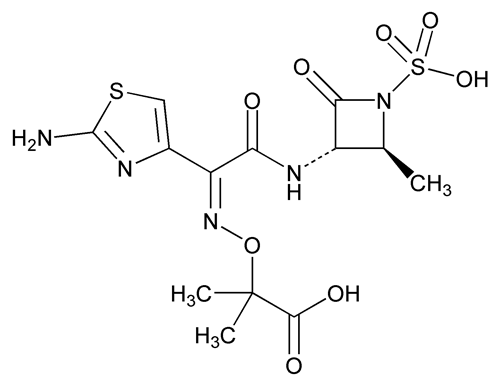
Aztreonam
C13H17N5O8S2 435.43
Propanoic acid, 2-[[[1-(2-amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methyl-, [2S-[2 ,3
,3 (Z)]]-;
(Z)]]-;
(Z)-2-[[[(2-Amino-4-thiazolyl)[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid

 [78110-38-0].
[78110-38-0].
(az tree' oh nam).
C13H17N5O8S2 435.43
Propanoic acid, 2-[[[1-(2-amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methyl-, [2S-[2
(Z)-2-[[[(2-Amino-4-thiazolyl)[[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid
DEFINITION
Aztreonam, which may be anhydrous or hydrated, contains NLT 92.0% and NMT 105.0% of C13H17N5O8S2, calculated on the anhydrous and solvent-free basis.
IDENTIFICATION
• Infrared Absorption  197K
197K :
If a difference appears in the IR spectra of the analyte and the standard, dissolve equal portions of the test specimen and the reference standard in equal volumes of methanol. [Note—To achieve a complete dissolution, it is suggested to use about 25 mL of methanol for each 50 mg of material, and stir the mixture for 40 min at room temperature. ]
:
If a difference appears in the IR spectra of the analyte and the standard, dissolve equal portions of the test specimen and the reference standard in equal volumes of methanol. [Note—To achieve a complete dissolution, it is suggested to use about 25 mL of methanol for each 50 mg of material, and stir the mixture for 40 min at room temperature. ]
Evaporate the solutions to dryness under vacuum, and dry at 40 for 4 h under vacuum. Perform the test on the residues.
for 4 h under vacuum. Perform the test on the residues.
ASSAY
Change to read:
• Procedure
[Note—Store the System suitability solution, Standard solution, and Sample solution at 5 , and protect from light to prevent isomerization of aztreonam Z-isomer to aztreonam E-isomer. ]
, and protect from light to prevent isomerization of aztreonam Z-isomer to aztreonam E-isomer. ]
Buffer:
6.8 mg/mL of monobasic potassium phosphate in water. Adjust with 1 M phosphoric acid to a pH of 3.0.
Mobile phase:
Methanol and Buffer (1:4)
System suitability solution:
1 mg/mL of USP Aztreonam RS and 1 mg/mL of USP Aztreonam E-Isomer RS in Mobile phase
Standard solution:
1 mg/mL of USP Aztreonam RS in Mobile phase
Sample solution:
1 mg/mL of Aztreonam in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:

 USP353.9-mm × 30-cm; 10-µm packing L1
USP353.9-mm × 30-cm; 10-µm packing L1
Flow rate:
1.5 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for aztreonam and aztreonam E-isomer are 1.0 and 1.8, respectively. ]
Suitability requirements
Resolution:
NLT 2.0 between aztreonam and aztreonam E-isomer, System suitability solution
Tailing factor:
NMT 2 for aztreonam, System suitability solution
Relative standard deviation:
NMT 2.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of aztreonam (C13H17N5O8S2) in the portion of Aztreonam taken:
Result = (rU/rS) × (CS/CU) × P × F × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Aztreonam RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Aztreonam in the Sample solution (mg/mL) |
| P | = | = potency of USP Aztreonam RS (µg/mg) |
| F | = | = unit conversion factor, 0.001 mg/µg |
Acceptance criteria:
92.0%–105.0% on the anhydrous and solvent-free basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%, the charred residue being moistened with 2 mL of nitric acid and 5 drops of sulfuric acid
:
NMT 0.1%, the charred residue being moistened with 2 mL of nitric acid and 5 drops of sulfuric acid
• Heavy Metals, Method II  231
231 :
NMT 30 ppm
:
NMT 30 ppm
Organic Impurities
• Procedure
[Note—Store the System suitability solution, Standard solution, and Sample solution at 5 , and protect from light to prevent isomerization of aztreonam Z-isomer to aztreonam E-isomer. ]
, and protect from light to prevent isomerization of aztreonam Z-isomer to aztreonam E-isomer. ]
Mobile phase, System suitability solution, Standard solution, Sample solution, Chromatographic system, and System suitability:
Proceed as directed in the Assay.
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Aztreonam taken:
Result = (rU/rS) × (CS/CU) × P × F × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of aztreonam from the Standard solution |
| CS | = | = concentration of USP Aztreonam RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Aztreonam in the Sample solution (mg/mL) |
| P | = | = potency of USP Aztreonam RS (µg/mg) |
| F | = | = unit conversion factor, 0.001 mg/µg |
Acceptance criteria
Individual impurities:
See Table 1.
Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Open-ring aztreonama and open-ring desulfated aztreonamb,c | 0.55 | 1.0 |
| Aztreonam (Z-isomer) | 1.0 | — |
| Desulfated aztreonamd | 1.6 | 1.5 |
| Aztreonam E-isomere | 1.8 | 0.5 |
| Aztreonam ethyl esterf | 3.9 | 1.5 |
| Any individual unspecified impurity | — | 0.1 |
| Total impurities | — | 3.0 |
|
a
(2S,3S)-2-{(Z)-2-[2-Aminothiazol-4-yl]-2-[2-carboxypropan-2-yloxyimino]acetamido}-3-(sulfoamino)butanoic acid.
b
(2S,3S)-3-Amino-2-{(Z)-2-[2-aminothiazol-4-yl]-2-[2-carboxypropan-2-yloxyimino]acetamido}butanoic acid.
c
Open-ring aztreonam and open-ring desulfated aztreonam coelute. The limit is for the sum of these two impurities.
d
(Z)-2-({[(2-Amino-4-thiazolyl){[(2S,3S)-2-methyl-4-oxo-3-azetidinyl]carbamoyl}methylene]amino}oxy)-2-methylpropionic acid.
e
(E)-2-({[(2-Amino-4-thiazolyl){[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl}methylene]amino}oxy)-2-methylpropionic acid.
f
Ethyl (Z)-2-({[(2-amino-4-thiazolyl){[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl}methylene]amino}oxy)-2-methylpropionate.
|
||
SPECIFIC TESTS
• Sterility Tests  71
71 :
Where the label states that Aztreonam is sterile, it meets the requirements for Test for Sterility of the Product to Be Examined—Membrane Filtration, using Fluid A, to which 23.4 g of sterile arginine has been added to each 1000 mL.
:
Where the label states that Aztreonam is sterile, it meets the requirements for Test for Sterility of the Product to Be Examined—Membrane Filtration, using Fluid A, to which 23.4 g of sterile arginine has been added to each 1000 mL.
• Water Determination, Method I  921
921 :
NMT 2.0%; if labeled as the hydrated form: 12.0%–18.0%. [Note—The term hydrated form refers to the
:
NMT 2.0%; if labeled as the hydrated form: 12.0%–18.0%. [Note—The term hydrated form refers to the  -form of Aztreonam, which is not a stoichiometric hydrate. ]
-form of Aztreonam, which is not a stoichiometric hydrate. ]
• Bacterial Endotoxins Test  85
85 :
Where the label states that aztreonam is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.17 USP Endotoxin Unit/mg of aztreonam.
:
Where the label states that aztreonam is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.17 USP Endotoxin Unit/mg of aztreonam.
• Limit of Alcohol
[Note—This test is to be performed if alcohol is used while manufacturing Aztreonam. ]
Standard solution:
0.004 mL/mL of alcohol from USP Alcohol Determination—Alcohol RS and 0.004 mL/mL of acetonitrile from USP Alcohol Determination—Acetonitrile RS in dimethylformamide. [Note—The Standard solution contains 0.4% alcohol and 0.4% acetonitrile. ]
Sample solution:
80 mg/mL of Aztreonam and 0.004 mL/mL of acetonitrile in dimethylformamide. [Note—Dissolve Aztreonam in dimethylformamide using 20% of the final volume. Add a suitable aliquot of USP Alcohol Determination—Acetonitrile RS, and dilute with dimethylformamide to volume. The concentration of acetonitrile in the Sample solution is 0.4%. ]
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.53-mm × 30-m; phase G43
Film thickness:
3.0 µm
Temperature
Injector:
210
Detector:
280
Column:
See Table 2.
Table 2
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 50 | 0 | 50 | 5 |
| 50 | 10 | 200 | 4 |
Carrier gas:
He
Linear velocity:
35 cm/s
Injection mode:
Split
Split ratio:
5:1
Injection size:
0.5 µL
System suitability
[Note—The relative retention times for alcohol and acetonitrile are 1.0 and 1.3, respectively. ]
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 2.0 between alcohol and acetonitrile
Tailing factor:
NMT 1.5
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of alcohol in the portion of Aztreonam taken:
Result = (RU/RS) × (CS × D/CU) × F × 100
| RU | = | = peak response ratio of alcohol to acetonitrile from the Sample solution |
| RS | = | = peak response ratio of alcohol to acetonitrile from the Standard solution |
| CS | = | = concentration of alcohol in the Standard solution (mL/mL) |
| D | = | = density of alcohol (g/mL) |
| CU | = | = concentration of Aztreonam in the Sample solution (mg/mL) |
| F | = | = unit conversion factor, 1000 mg/g |
Acceptance criteria:
NMT 4%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• Labeling:
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms. Where it is the hydrated form, the label so indicates.
• USP Reference Standards  11
11
USP Alcohol Determination—Acetonitrile RS
C2H3N 41.05
C2H3N 41.05
USP Alcohol Determination—Alcohol RS
C2H5OH 46.07
C2H5OH 46.07
USP Aztreonam RS 
Propanoic acid, 2-[[[1-(2-amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methyl-, [2S-[2 ,3
,3 (Z)]]-.
(Z)]]-.
C13H17N5O8S2 435.43

Propanoic acid, 2-[[[1-(2-amino-4-thiazolyl)-2-[(2-methyl-4-oxo-1-sulfo-3-azetidinyl)amino]-2-oxoethylidene]amino]oxy]-2-methyl-, [2S-[2
C13H17N5O8S2 435.43
USP Aztreonam E-Isomer RS 
(E)-2-({[(2-Amino-4-thiazolyl){[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl}methylene]amino}oxy)-2-methylpropionic acid.
C13H17N5O8S2 435.43

(E)-2-({[(2-Amino-4-thiazolyl){[(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl}methylene]amino}oxy)-2-methylpropionic acid.
C13H17N5O8S2 435.43
USP Endotoxin RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2289
Pharmacopeial Forum: Volume No. 36(6) Page 1496