Polyethylene Oxide
(pol'' ee eth' i leen ox' ide).
DEFINITION
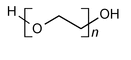
Polyethylene Oxide is a nonionic homopolymer of ethylene oxide, represented:
H(OCH2CH2)nOH
in which n represents the average number of oxyethylene groups. The number n varies from about 2000 to 200,000. It is a white to off-white powder obtainable in several grades, varying in viscosity profile in an aqueous isopropyl alcohol solution. It may contain a suitable antioxidant.
IDENTIFICATION
• A. Infrared Absorption  197K
197K
Sample:
Previously dried in a vacuum at room temperature to constant weight
• B. Procedure
Viscosity
[Note—Based on Labeling information, perform the following test accordingly. ]
Analysis:
Pass Polyethylene Oxide through a 20-mesh screen. Then transfer Polyethylene Oxide to a 800-mL low-form beaker the amount which is specified in Table 1 to provide the solution concentration.
Table 1
| Sample solution | |||
|---|---|---|---|
| Polyethylene oxide weight | 1% Solution | 2% Solution | 5% Solution |
| 6 g | 12 g | 30 g | |
Add 125 mL of dehydrated isopropyl alcohol to the beaker. Place the stirrer into the beaker with an appropriate glass cover. Stir the polyethylene oxide-isopropanol mixture at a rate to ensure that a slurry is formed. Add the prescribed amount of water to the polyethylene oxide-isopropanol slurry, refer to Table 2. All solution concentrations are based on the water content of the aqueous isopropyl alcohol solution. Be careful to avoid splashing of the water. Adjust the temperature to near 25 to assist the final solution coming to temperature.
to assist the final solution coming to temperature.
Table 2
| Sample solution | |||
|---|---|---|---|
| Water weight | 1% Solution | 2% Solution | 5% Solution |
| 594 g | 588 g | 570 g | |
Ensure that the stirring is very effective at the beginning for about 1 min. Then allow to gently stir for at least 3 h. Ensure that Polyethylene Oxide dissolves in solution, take care to avoid mixing in excess air, and stop the stirring. [Note—Ensure a colloidal dispersion by stirring for at least 3 h if added antioxidant or silicon dioxide is not soluble in the system. ]
Place a watchglass over the beaker and place in a bath for at least 30 min. When the solution reaches 25 ± 0.1 , determine the viscosity of the Sample solution using the viscometer, spindle, and speed indicated on the Labeling. [Note—A guard may be required as indicated on the Labeling. ] Follow the instrument manufacturer’s directions to measure the apparent viscosity.
, determine the viscosity of the Sample solution using the viscometer, spindle, and speed indicated on the Labeling. [Note—A guard may be required as indicated on the Labeling. ] Follow the instrument manufacturer’s directions to measure the apparent viscosity.
Acceptance criteria:
Viscosity falls within the viscosity range indicated by the Labeling.
IMPURITIES
Inorganic Impurities
• Heavy Metals, Method II  231
231 :
NMT 10 ppm
:
NMT 10 ppm
• Silicon Dioxide and Nonsilicon Dioxide Residue on Ignition
Sample:
1 g
Analysis:
Weigh the Sample into a previously ignited, tared 50-mL platinum crucible. Add 4 drops of sulfuric acid. Heat carefully on a hot plate until the specimen is thoroughly charred and fumes no longer are evolved. Ignite the crucible at 700 ± 25 (see Residue on Ignition
(see Residue on Ignition  281
281 ) to constant weight. Wet the residue carefully with 1 mL of water, and slowly add 20 drops of hydrofluoric acid. [Caution—Hydrofluoric acid is an extremely hazardous chemical. When handling it, wear a face shield, arm protection, and rubber gloves, and perform the operation in a hood.
] Evaporate slowly on a hot plate to dryness, then ignite at 700 ± 25
) to constant weight. Wet the residue carefully with 1 mL of water, and slowly add 20 drops of hydrofluoric acid. [Caution—Hydrofluoric acid is an extremely hazardous chemical. When handling it, wear a face shield, arm protection, and rubber gloves, and perform the operation in a hood.
] Evaporate slowly on a hot plate to dryness, then ignite at 700 ± 25 for 10 min, cool to room temperature in a desiccator, and weigh. Repeat the addition of hydrofluoric acid, evaporation, and ignition, to constant weight.
for 10 min, cool to room temperature in a desiccator, and weigh. Repeat the addition of hydrofluoric acid, evaporation, and ignition, to constant weight.
Calculate the percentage of silicon dioxide residue on ignition from the difference between the net weights before and after the hydrofluoric acid treatment.
Calculate the percentage of nonsilicon dioxide residue on ignition from the final net weight.
Acceptance criteria
Silicon dioxide residue on ignition:
NMT 3%
Nonsilicon dioxide residue on ignition:
NMT 2%
Organic Impurities
• Procedure: Limit of Free Ethylene Oxide
Standard stock solution:
[Caution—Ethylene oxide is toxic and flammable. Prepare solutions of it in a well-ventilated fume hood.
] Prepare the solution using the special handling described below. Ethylene oxide is a gas at room temperature. It is usually stored in a lecture-type gas cylinder or small metal pressure bomb. Chill the cylinder in a refrigerator before use. Transfer 5 mL of the liquid ethylene oxide to a cold, 10-mL serum vial. Seal the vial, and store in a refrigerator. Transfer 40 g of acetone to a tared 50-mL serum vial that is capable of being tightly sealed with a polytef-lined septum and a metallic crimp cap. Seal the vial, and weigh it. Using a gas-tight gas chromatographic syringe that has been chilled in a refrigerator, transfer 60 µL of the liquefied ethylene oxide to the same vial. Weigh the vial, and determine the amount added by weight difference. The solution contains about 1 µg/µL of ethylene oxide. [Note—This solution may be kept for 1 week in the crimp-sealed serum vial, stored in a freezer. ]
[Note—Standard solutions A–D and the Sample solution should be prepared in vials designed for use in the headspace sampling system specified in the Chromatographic system. ]
Standard solution A:
To a tared vial add 1.0 g of Polyethylene Oxide, and seal the vial. Through the septum add 2.0 µL of Standard stock solution, heat the vial at 100 for 30 min, and cool to room temperature.
for 30 min, and cool to room temperature.
Standard solution B:
To a tared vial add 1.0 g of Polyethylene Oxide, and seal the vial. Through the septum add 4.0 µL of Standard stock solution, heat the vial at 100 for 30 min, and cool to room temperature.
for 30 min, and cool to room temperature.
Standard solution C:
To a tared vial add 1.0 g of Polyethylene Oxide, and seal the vial. Through the septum add 6.0 µL of Standard stock solution, heat the vial at 100 for 30 min, and cool to room temperature.
for 30 min, and cool to room temperature.
Standard solution D:
To a tared vial add 1.0 g of Polyethylene Oxide, and seal the vial. Through the septum add 8.0 µL of Standard stock solution, heat the vial at 100 for 30 min, and cool to room temperature.
for 30 min, and cool to room temperature.
Sample solution:
To a tared vial add 1.0 g of Polyethylene Oxide, and seal the vial. Heat the vial at 100 for 30 min, and cool to room temperature.
for 30 min, and cool to room temperature.
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.53-mm × 10-m capillary column bonded with a 20-µm layer of phase G45
Temperature
Injector:
200
Detector:
250
Column:
See the temperature program table below.
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 70 | — | 70 | 5 |
| 70 | 10 | 200 | 5 |
Flow rate:
15 mL/min
Injection size:
300 µL of headspace gas
Injection type:
Split
Carrier gas:
Helium
[Note—The makeup gas is also helium, with a split flow rate of 15 mL/min. ]
System suitability
Samples:
Standard stock solution and Standard solution C
Suitability requirements
Relative standard deviation:
NMT 5%. [Note—Multiple vials are prepared for replicate injections. ]
Analysis
Samples:
Standard solutions A–D and Sample solution
[Note—A headspace apparatus that automatically transfers the measured amount of gaseous headspace may be used to perform the injections. ]
Using a gas-tight syringe, separately inject equal volumes (about 300 µL) of the gaseous headspace of each of the Standard solutions and the Sample solution into the gas chromatograph, record the chromatograms, and measure the areas of the peak responses. Determine by a retention time comparison whether ethylene oxide is detected in the Sample solution. Plot the responses of the Sample solution and the Standard solutions versus the content, in µg, of ethylene oxide in each vial, as furnished by the Standard stock solution. Draw the straight line best fitting the five points, and calculate the correlation coefficient for the line.
[Note—The content of ethylene oxide, as furnished by the Standard stock solution, is 0 µg in the Sample solution. ]
A suitable system is one that yields a line having a correlation coefficient of NLT 0.99. Extrapolate the line until it intercepts the content axis on the negative side. From the intercept, determine the total amount, TU, in µg, of ethylene oxide in the Sample solution.
Calculate the percentage of ethylene oxide in the portion of Polyethylene Oxide taken:
Result = (TU/W) × 100
| TU | = | = total amount of ethylene oxide in the Sample solution (µg) |
| W | = | = weight of Polyethylene Oxide taken to prepare the Sample solution (µg) |
Acceptance criteria:
NMT 0.001%
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry 4 g at 105
:
Dry 4 g at 105 for 45 min: it loses NMT 1.0% of its weight.
for 45 min: it loses NMT 1.0% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers. No storage requirements specified.
• Labeling:
Label it to indicate the viscosity and acceptable limits, giving the viscosity measurement parameters, concentration of the solution, and the type of equipment used. Label it to indicate the name and quantity of any added antioxidant.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1906
Pharmacopeial Forum: Volume No. 36(1) Page 191