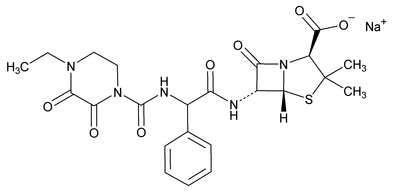
Piperacillin Sodium
C23H26N5NaO7S 539.54
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino]phenylacetyl]amino]-3,3-dimethyl-7-oxo-, monosodium salt, [2S-[2 ,5
,5 ,6
,6 (S*)]];
(S*)]];
Sodium (2S,5R,6R)-6-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate

 [59703-84-3].
[59703-84-3].
(pi'' per a sil' in soe' dee um).
C23H26N5NaO7S 539.54
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino]phenylacetyl]amino]-3,3-dimethyl-7-oxo-, monosodium salt, [2S-[2
Sodium (2S,5R,6R)-6-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate
DEFINITION
Piperacillin Sodium has a potency equivalent to NLT 863 µg/mg and NMT 1007 µg/mg of piperacillin (C23H27N5O7S), calculated on the anhydrous basis.
IDENTIFICATION
• A.
The retention time of the major peak from the Sample solution corresponds to that from the Standard solution, and the chromatogram compares qualitatively to that from the Standard solution in the Assay.
ASSAY
• Procedure
Mobile phase:
Methanol, water, 0.2 M monobasic sodium phosphate, and 0.4 M tetrabutylammonium hydroxide(450:447:100:3). Adjust with phosphoric acid to a pH of 5.50 ± 0.02.
System suitability solution:
0.1 mg/mL of USP Ampicillin RS and 0.2 mg/mL of USP Piperacillin RS in Mobile phase
Standard solution 1:
0.4 mg/mL of USP Piperacillin RS in Mobile phase. Dissolve in a few drops of methanol, and dilute with Mobile phase to volume. Use this solution within 1 h.
Sample solution:
0.4 mg/mL of Piperacillin Sodium in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 25-cm; packing L1
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—See Table 1 for relative retention times. ]
Suitability requirements
Resolution:
NLT 16 between ampicillin and piperacillin, System suitability solution
Tailing factor:
NMT 1.2 for the piperacillin peak, System suitability solution
Relative standard deviation:
NMT 2% for the piperacillin peak, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the potency, in µg/mg, of piperacillin (C23H27N5O7S) in the portion of Piperacillin Sodium taken:
Result = (rU/rS) × (CS/CU) × P
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Piperacillin RS in the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
| P | = | = potency of piperacillin in USP Piperacillin RS (µg/mg) |
Acceptance criteria:
863–1007 µg/mg on the anhydrous basis
IMPURITIES
• Piperacillin Related Compounds A and C
Mobile phase, Standard solution 1, and Sample solution:
Prepare as directed in the Assay.
System suitability solution:
0.1 mg/mL of USP Ampicillin RS and 0.2 mg/mL of USP Piperacillin RS in Mobile phase
Standard solution 2:
0.04 mg/mL of USP Piperacillin RS in Mobile phase. Dissolve in a few drops of methanol, and dilute with Mobile phase to volume. Use this solution within 1 h.
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 25-cm; packing L1
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Samples:
Standard solution 1 and System suitability solution
[Note—See Table 1 for relative retention times. ]
Suitability requirements
Resolution:
NLT 16 between ampicillin and piperacillin, System suitability solution
Tailing factor:
NMT 1.2 for the piperacillin peak, System suitability solution
Relative standard deviation:
NMT 2% for the piperacillin peak, Standard solution 1
Analysis
Samples:
Standard solution 2 and Sample solution
Calculate the percentages of piperacillin related compounds A and C in the portion of Piperacillin Sodium taken:
Result = (rU/rS) × (CS/CU) × P × F1 × F2 × 100
| rU | = | = peak response of piperacillin related compound A or C from the Sample solution |
| rS | = | = peak response of piperacillin from the Standard solution 2 |
| CS | = | = concentration of USP Piperacillin RS in the Standard solution 2 (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
| P | = | = potency of piperacillin in USP Piperacillin RS (µg/mg) |
| F1 | = | = relative response factor (see Table 1) |
| F2 | = | = conversion factor, 0.001 mg/µg |
Acceptance criteria:
See Table 1.
Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Piperacillin related compound B | 0.24 | — | — |
| Ampicillin | 0.31 | — | — |
| Piperacillin related compound C | 0.37 | 0.93 | 1.0 |
| Piperacillin related compound A | 0.62 | 1.4 | 3.5 |
| Piperacillin | 1.0 | — | — |
SPECIFIC TESTS
• Water Determination, Method I  921
921
Test preparation:
Proceed as described for hygroscopic substances.
Acceptance criteria:
NMT 1.0%
• Bacterial Endotoxins Test  85
85 :
Where the label states that Piperacillin Sodium is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.07 USP Endotoxin Unit/mg of piperacillin.
:
Where the label states that Piperacillin Sodium is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.07 USP Endotoxin Unit/mg of piperacillin.
• Sterility Tests  71
71 :
Where the label states that Piperacillin Sodium is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements when tested as directed under Test for Sterility of the Product to Be Examined, Membrane Filtration.
:
Where the label states that Piperacillin Sodium is sterile or that it must be subjected to further processing during the preparation of injectable dosage forms, it meets the requirements when tested as directed under Test for Sterility of the Product to Be Examined, Membrane Filtration.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• Labeling:
Where it is intended for use in preparing injectable dosage forms, the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4333