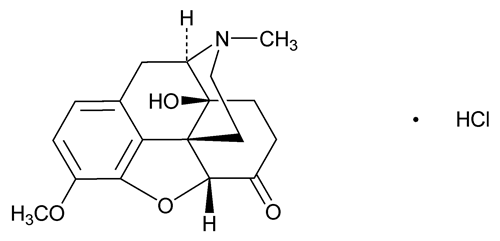
Oxycodone Hydrochloride
C18H21NO4·HCl 351.82
Morphinan-6-one, 4,5-epoxy-14-hydroxy-3-methoxy-17-methyl-, hydrochloride, (5 )-;
)-;
4,5 -Epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one hydrochloride
-Epoxy-14-hydroxy-3-methoxy-17-methylmorphinan-6-one hydrochloride


 [124-90-3].
[124-90-3].
(ox'' i koe' done hye'' droe klor' ide).
C18H21NO4·HCl 351.82
Morphinan-6-one, 4,5-epoxy-14-hydroxy-3-methoxy-17-methyl-, hydrochloride, (5
4,5
DEFINITION
Oxycodone Hydrochloride contains NLT 97.0% and NMT 103.0% of C18H21NO4·HCl, calculated on the anhydrous, solvent-free basis.
IDENTIFICATION
• A. Procedure
Sample solution:
Dissolve 250 mg in 25 mL of water.
Analysis:
Render the 25 mL of Sample solution alkaline with 6 N ammonium hydroxide. Allow the mixture to stand until a precipitate is formed. Filter, wash the precipitate with 50 mL of cold water, and dry for 2 h at 105 .
.
Acceptance criteria:
The precipitate so obtained melts between 218 and 223
and 223 , but the range between the beginning and the end of melting does not exceed 2
, but the range between the beginning and the end of melting does not exceed 2 (see Melting Range or Temperature
(see Melting Range or Temperature  741
741 ).
).
• B. Infrared Absorption  197K
197K :
Use a portion of the dried precipitate obtained in Identification test A.
:
Use a portion of the dried precipitate obtained in Identification test A.
ASSAY
• Procedure
Mobile phase:
0.005 M sodium 1-hexanesulfonate, methanol, triethylamine, and phosphoric acid (900:100:2:5). Adjust with 50% sodium hydroxide solution to a pH of 2.5 ± 0.1, and filter.
System suitability solution:
13 µg/mL of codeine phosphate and 9 µg/mL of oxycodone in Mobile phase
Standard solution:
0.9 mg/mL of USP Oxycodone RS in Mobile phase
Sample solution:
1 mg/mL of Oxycodone Hydrochloride in Mobile phase. [Note—Pass a portion of this solution through a filter having a 0.5-µm or finer pore size, and use the filtrate as the Sample solution. ]
Chromatographic system
Mode:
LC
Detector:
UV 206 nm
Column:
3.9-mm × 15-cm; 4-µm packing L7
Column temperature:
50
Flow rate:
1.5 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for codeine and oxycodone are about 0.8 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 3.0 between codeine and oxycodone, System suitability solution
Tailing factor:
0.75–1.25, Standard solution
Relative standard deviation:
NMT 2.0% from replicate injections, Standard solution
Analysis
Samples:
Standard solution and Sample solution
[Note—Record the chromatograms for a period of time that is twice the retention time of the main oxycodone peak. ]
Calculate the percentage of C18H21NO4·HCl in the portion of Oxycodone Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Oxycodone RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Oxycodone Hydrochloride in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of oxycodone hydrochloride, 351.82 |
| Mr2 | = | = molecular weight of oxycodone base, 315.37 |
Acceptance criteria:
97.0%–103.0% on the anhydrous, solvent-free basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.05%. [Note—Use of sulfuric acid is omitted. ]
:
NMT 0.05%. [Note—Use of sulfuric acid is omitted. ]
Organic Impurities
• Procedure 1: Limit of Alcohol
Internal standard stock solution:
Transfer 6.0 mL of isopropyl alcohol to a 500-mL volumetric flask, and dilute with water to volume. [Note—The isopropyl alcohol must be free of alcohol impurities. ]
Internal standard solution:
Transfer 5.0 mL of the Internal standard stock solution to a 100-mL volumetric flask, and dilute with water to volume.
Standard stock solution:
16 mg/mL of alcohol (C2H5OH) in water
Standard solution:
Pipet 3.0 mL of the Standard stock solution and 5.0 mL of the Internal standard stock solution into a 100-mL volumetric flask, and dilute with water to volume.
Sample solution:
Transfer about 240 mg of Oxycodone Hydrochloride to a 15-mL centrifuge tube, add 5.0 mL of the Internal standard solution, and mix to dissolve.
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
4-mm × 1.8-m glass; packed with 80- to 100-mesh support S3
Carrier gas:
Helium
Temperature
Column:
150 . [Note—Condition the column overnight at 235
. [Note—Condition the column overnight at 235 with a slow flow of carrier gas. ]
with a slow flow of carrier gas. ]
Injector:
170
Detector:
170
Injection size:
5 µL
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 2 between isopropyl alcohol and alcohol
Tailing factor:
NMT 1.5
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of alcohol (C2H5OH) in the portion of Oxycodone Hydrochloride taken:
Result = (RU/RS) × (CS/CU) × 100
| RU | = | = peak response ratio of the alcohol peak to the isopropyl alcohol from the Sample solution |
| RS | = | = peak response ratio of the alcohol peak to the isopropyl alcohol from the Standard solution |
| CS | = | = concentration of alcohol in the Standard solution (mg/mL) |
| CU | = | = concentration of Oxycodone Hydrochloride in the Sample solution (mg/mL) |
Acceptance criteria:
NMT 1.0%
• Procedure 2
Analysis:
Use the chromatogram of the Sample solution in the Assay to calculate the percentage of each impurity in the portion of Oxycodone Hydrochloride taken:
Result = (rU/rT) × 100
| rU | = | = peak response for each impurity |
| rT | = | = sum of the responses of all the peaks |
Acceptance criteria
Individual impurities:
The impurities meet the requirements listed in Impurity Table 1.
Total impurities:
NMT 2.0%
Impurity Table 1
| Name | Relative Retention Time | Acceptance Criteria, NMT (%) |
|---|---|---|
| Oxymorphone | 0.31 | 0.15 |
| Noroxymorphone | 0.33 | 0.15 |
| 10-Hydroxyoxycodone | 0.53 | 0.15 |
| 6- |
0.67 | 0.25 |
| 7,8-Dihydro-8 |
0.71 | 0.15 |
| Hydrocodone | 1.19 | 0.15 |
| Individual unspecified impurity | — | 0.10 |
• Procedure 3: Limit of Oxycodone Related Compound A (14-Hydroxycodeinone) and Oxycodone Related Compound C (Codeinone)
Solution A:
Dissolve 3.45 g of monobasic sodium phosphate in 1000 mL of water. Add 5.41 g of sodium dodecyl sulfate, and mix. Filter, and adjust with 50% (w/v) sodium hydroxide solution to a pH of 7.50 ± 0.05.
Solution B:
Water and phosphoric acid (9:1)
Diluent:
Prepare a mixture of water and Solution B (9:1).
Mobile phase:
Prepare a mixture of acetonitrile, methanol, and Solution A (15.8:12.0:72.2), and adjust with Solution B to a pH of 7.80 ± 0.01.
Standard solution:
50 mg/mL of USP Oxycodone Hydrochloride RS and 0.5 µg/mL each of USP Oxycodone Related Compound A RS and USP Oxycodone Related Compound C RS in Diluent
Unspiked oxycodone hydrochloride solution:
50 mg/mL of USP Oxycodone Hydrochloride RS in Diluent
System suitability solution:
100 µg/mL of USP Oxycodone Hydrochloride RS and 5 µg/mL each of USP Oxycodone Related Compound A RS and USP Oxycodone Related Compound C RS in Diluent
Sample solution:
50 mg/mL of Oxycodone Hydrochloride in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
3.0-mm × 15-cm; 3.5-µm packing L1
Column temperature:
40
Flow rate:
0.7 mL/min
Injection size:
5 µL
System suitability
Samples:
Standard solution and System suitability solution
[Note—The relative retention times for oxycodone related compound C, oxycodone related compound A, and oxycodone are about 0.44, about 0.85, and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 4 between oxycodone related compound A and oxycodone related compound C, System suitability solution
Tailing factor:
NMT 2.0, System suitability solution
Relative standard deviation:
NMT 20% for oxycodone related compound A and C, Standard solution
Analysis
Samples:
Diluent, Standard solution, Unspiked oxycodone hydrochloride solution, and Sample solution
Calculate the percentage of oxycodone related compound A and oxycodone related compound C in the portion of Oxycodone Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of oxycodone related compound A or oxycodone related compound C from the Sample solution |
| rS | = | = peak response of oxycodone related compound A or oxycodone related compound C minus the response of the Unspiked oxycodone hydrochloride solution from the Standard solution |
| CS | = | = concentration of USP Oxycodone Related Compound A RS or USP Oxycodone Related Compound C RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Oxycodone Hydrochloride in the Sample solution (mg/mL) |
Acceptance criteria
Individual impurities:
See Impurity Table 2.
(Procedure 3 postponed indefinitely)
SPECIFIC TESTS
• Content of Chloride
Sample solution:
6 mg/mL in methanol
Analysis:
To 50 mL of Sample solution add 5 mL of glacial acetic acid, and titrate with 0.1 N silver nitrate VS, determining the endpoint potentiometrically. Each mL of 0.1 N silver nitrate is equivalent to 3.545 mg of Cl.
Acceptance criteria:
9.8%–10.4% calculated on the anhydrous, solvent-free basis
• Optical Rotation, Specific Rotation  781S
781S :
:
 137
137 to
to  149
149
Sample solution:
25 mg/mL of Oxycodone Hydrochloride in water, on the anhydrous, solvent-free basis
• Water Determination, Method I  921
921 :
NMT 7.0%
:
NMT 7.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• USP Reference Standards  11
11
USP Oxycodone Related Compound A RS
14-Hydroxycodeinone.
14-Hydroxycodeinone.
USP Oxycodone Related Compound C RS
Codeinone.
Codeinone.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4170
Pharmacopeial Forum: Volume No. 34(6) Page 1480