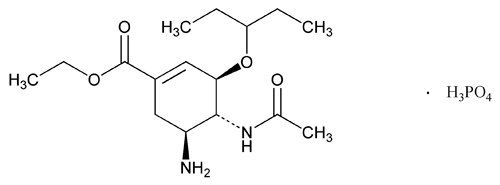
Oseltamivir Phosphate
C16H28N2O4·H3PO4 410.40
[3R-(3 ,4
,4 ,5
,5 )]-Ethyl 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (1:1);
)]-Ethyl 4-(acetylamino)-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (1:1);
Ethyl (3R,4R,5S)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate, phosphate (1:1)

 [204255-11-8].
[204255-11-8].
(oh'' sel tam' i vir fos' fate).
C16H28N2O4·H3PO4 410.40
[3R-(3
Ethyl (3R,4R,5S)-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate, phosphate (1:1)
DEFINITION
Oseltamivir Phosphate contains NLT 98.0% and NMT 101.5% of C16H28N2O4·H3PO4, calculated on the anhydrous basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
Dissolve 6.8 g of potassium dihydrogen phosphate in 980 mL of water. Adjust with 1 M potassium hydroxide solution to a pH of 6.0, and dilute with water to 1 L.
Mobile phase:
Methanol, acetonitrile, and Solution A (245:135:620)
Diluent:
Methanol, acetonitrile, and water (245:135:620)
Standard solution:
1 mg/mL of USP Oseltamivir Phosphate RS in Diluent
Sample solution:
1 mg/mL of Oseltamivir Phosphate in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 207 nm
Column:
4.6-mm × 25-cm; packing L7
Column temperature:
50
Flow rate:
1.2 mL/min
Injection size:
15 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 2.0
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C16H28N2O4 ·H3PO4 in the portion of Oseltamivir Phosphate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Oseltamivir Phosphate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Oseltamivir Phosphate in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–101.5% on the anhydrous basis
IMPURITIES
Inorganic Impurities
• Heavy Metals  231
231 :
NMT 10 ppm
:
NMT 10 ppm
Organic Impurities
• Procedure 1
Solution A, Mobile phase, Diluent, Standard solution, Sample solution, and Chromatographic system:
Proceed as directed in the Assay.
Analysis
Sample:
Sample solution
Calculate the percentage of each impurity in the portion of Oseltamivir Phosphate taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response of each individual impurity from the Sample solution |
| rS | = | = peak response of oseltamivir phosphate from the Standard solution |
| CS | = | = concentration of USP Oseltamivir Phosphate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Oseltamivir Phosphate in the Sample solution (mg/mL) |
| F | = | = relative response factor from Impurity Table 1 |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.7%
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Oseltamivir acida | 0.17 | 1.4 | 0.3 |
| Oseltamivir phenolb |
0.51 | 2.7 | 0.1 |
| Oseltamivir phosphate | 1.00 | 1.0 | — |
| Unspecified impurity |
— | 1.0 | 0.1 |
| Total unspecified impurity | — | — | 0.4 |
|
a
(3R,4R,5S)-4-Acetylamino-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylic acid.
b
4-Acetylamino-3-hydroxybenzoic acid ethyl ester.
|
|||
• Procedure 2: Oseltamivir Related Compound A
Buffer:
1.54 g/L of ammonium acetate in water
Mobile phase:
Acetonitrile, water, and Buffer (3:6:1)
Stock solution A:
50 µg/mL of USP Oseltamivir Related Compound A RS, prepared as follows: Dissolve in alcohol, using 5% of final volume, and dilute with water to volume.
Solution A:
1 µg/mL of USP Oseltamivir Related Compound A RS in water from Stock solution A
Standard solution:
10 mg/mL of USP Oseltamivir Phosphate RS in Solution A
Sample solution:
10 mg/mL of Oseltamivir Phosphate in water
Chromatographic system
Mode:
LC
Detectors:
UV 210 nm and mass spectrometer
Column:
3.0-mm × 5-cm; 5-µm packing L1
Flow rate:
1.5 mL/min
Injection size:
1 µL
Temperature:
40
Use electrospray (+) ionization, a selected ion monitoring mode with m/z of 356.2 (protonated oseltamivir related compound A). Adjust the dwell time, fragmentation voltage, drying gas temperature, drying gas flow, nebulizer pressure, and capillary voltage as appropriate for an optimal response. [Note—A postcolumn flow splitter with a split ratio of about 3:1 is used. ]
System suitability
Sample:
Standard solution
[Note—The relative retention time for oseltamivir related compound A versus oseltamivir is about 2.6. ]
Suitability requirements
Resolution:
The oseltamivir related compound A peak (detected by MD-SIM mode) and the oseltamivir peak (detected by UV) are baseline resolved.
[Note—The resolution of the two components minimizes background noise and ion suppression effects for the trace of oseltamivir related compound A by the oseltamivir matrix. ]
Relative standard deviation:
NMT 15.0%, oseltamivir related compound A peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of oseltamivir related compound A in the portion of Oseltamivir Phosphate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of oseltamivir related compound A from the Sample solution |
| rS | = | = peak response of oseltamivir related compound A from the Standard solution |
| CS | = | = concentration of USP Oseltamivir Related Compound A RS in the Standard solution (mg/mL) |
| CU | = | = concentration of oseltamivir phosphate in the Sample solution (mg/mL) |
Acceptance criteria:
NMT 0.01%
• Procedure 3: Limit Of Tributyl Phosphine Oxide
Blank:
Transfer 1.0 mL of suitable derivatizing reagent1 to a vial. Close the vial, shake, and heat for 20 min at 60 . Centrifuge the pyridinium salt precipitate.
. Centrifuge the pyridinium salt precipitate.
Standard stock solution 1:
21 mg/mL of USP Tributyl Phosphine Oxide RS in pyridine
Standard stock solution 2:
21 mg/mL of USP Oseltamivir Phosphate RS in suitable derivatizing reagent. Close the vial, mix, and heat for 20 min at 60 . Centrifuge the pyridinium salt precipitate.
. Centrifuge the pyridinium salt precipitate.
Standard solution:
21 µg/mL each of USP Tributyl Phosphine Oxide RS and USP Oseltamivir Phosphate RS in pyridine from Standard stock solution 1 and Standard stock solution 2, respectively
Sample solution:
Transfer 15 mg of Oseltamivir Phosphate to a vial. Add 1.0 mL of suitable derivatizing reagent. Close the vial, mix, and heat for 20 min to 60 . Centrifuge the pyridinium salt precipitate.
. Centrifuge the pyridinium salt precipitate.
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.32-mm × 30-m capillary column coated with a 0.25-µm phase G1
Split ratio:
1:50
Split flow:
64 mL/min
Injection size:
1 µL
Temperature
Detector:
260
Injection port:
260
Column:
See the temperature program table below.
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 180 | 0 | 180 | 2 |
| 180 | 8 | 250 | 10 |
Linear velocity:
27 cm/s
Carrier gas:
Helium
System suitability
Sample:
Standard solution
[Note—The relative retention times for tributyl phosphine oxide and oseltamivir phosphate are about 0.54 and 1.00, respectively. ]
Suitability requirements
Relative standard deviation:
NMT 10.0% for the tributyl phosphine oxide and osteltamivir phosphate peaks
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of tributyl phosphine oxide in the portion of Oseltamivir Phosphate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of tributyl phosphine oxide from the Sample solution |
| rS | = | = peak response of tributyl phosphine oxide from the Standard solution |
| CS | = | = concentration of tributyl phosphine oxide in the Standard solution (mg/mL) |
| CU | = | = concentration of Oseltamivir Phosphate in the Sample solution (mg/mL) |
Acceptance criteria:
NMT 0.1%
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
NMT 0.5%
:
NMT 0.5%
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
10 mg/mL in water
Acceptance criteria:
Between –30.7 and –32.6
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
• USP Reference Standards  11
11
USP Oseltamivir Related Compound A RS
(3S,4R,5S)-Ethyl 4-acetamido-5-amino-2-azido-3-(pentan-3-yloxy)cyclohexanecarboxylate.
C16H29N5O4 355.43
(3S,4R,5S)-Ethyl 4-acetamido-5-amino-2-azido-3-(pentan-3-yloxy)cyclohexanecarboxylate.
C16H29N5O4 355.43
USP Tributyl Phosphine Oxide RS
C12H27OP 218.32
C12H27OP 218.32
1
Tri-Sil Reagent (product number: 48999 0049001) may be obtained from Pierce: www.piercenet.com.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Leonel M. Santos, Ph.D.
Senior Scientific Liaison 1-301-816-8168 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4138
Pharmacopeial Forum: Volume No. 36(1) Page 124