Add the following:
(or' li stat).
C29H53NO5 495.73
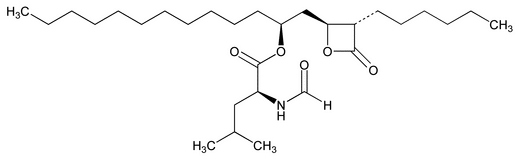
l-Leucine, N-formyl-, 1-[(3-hexyl-4-oxo-2-oxetanyl)methyl]dodecyl ester, [2S-[2
N-Formyl-l-leucine, ester with (3S,4S)-3-hexyl-4-[(2S)-2-hydroxytridecyl]-2-oxetanone
DEFINITION
Orlistat contains NLT 98.0% and NMT 101.5% of C29H53NO5, calculated on the anhydrous, solvent-free basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
[Note—Avoid the use of plastic flasks for the preparation or containment of any solution in this analysis. ]
Mobile phase:
Acetonitrile, phosphoric acid, and water (860: 0.05: 140)
Standard solution:
0.5 mg/mL of USP Orlistat RS in Mobile phase. Inject immediately after preparation or store at 5 .
.
Sample solution:
0.5 mg/mL of Orlistat in Mobile phase. Inject immediately after preparation or store at 5 .
.
Chromatographic system
Mode:
LC
Detector:
UV 195
Column:
3.9-mm × 15-cm; 4-µm packing L1
Flow rate:
1.0 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of orlistat (C29H53NO5) in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Orlistat RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Orlistat in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–101.5% on the anhydrous, solvent-free basis
IMPURITIES
Organic Impurities
• Procedure 1: Limit of Orlistat Related Compound A
Standard solution:
0.1 mg/mL of USP Orlistat Related Compound A RS in acetone
Sample solution:
50 mg/mL of Orlistat in acetone
Chromatographic system
Mode:
TLC
Adsorbent:
0.25-mm layer of chromatographic silica gel mixture
Application volume:
10 µL
Developing solvent system:
Toluene and ethyl acetate (4:1)
Detection solution:
Transfer 2.5 g of phosphomolybdic acid and 1 g of cerric sulfate into a 100-mL volumetric flask, dissolve in and dilute with methanol to volume.
Analysis
Samples:
Standard solution and Sample solution
Remove the plate, and air-dry it thoroughly. Spray the dried plate with Detection solution, and place the plate in an oven at 120 for 30 min.
for 30 min.
Acceptance criteria:
Any secondary spot from the Sample solution corresponding to orlistat related compound A is not more intense than the corresponding spot from the Standard solution (0.2%).
• Procedure 2: Limit of Orlistat Related Compound B
Standard solution:
0.025 mg/mL of USP Orlistat Related Compound B RS in methylene chloride
Sample solution:
50 mg/mL of Orlistat in methylene chloride
Spiked sample solution:
50 mg/mL of Orlistat in Standard solution
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.32-mm x 30-m fused silica, coated with a 0.25-µm G27 stationary phase
Column temperature:
See the temperature program table below.
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 50 | 4 | 170 | — |
| 170 | 30 | 300 | 30 |
Temperature
Injector:
270
Detector:
280
Carrier gas:
Helium
Flow rate:
30 mL/min
Split ratio:
10:1
Injection size:
2 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 10.0%
Analysis
Samples:
Sample solution and Spiked sample solution
Calculate the percentage of orlistat related compound B in the portion of Orlistat taken:
Result = [rU/(rSP  rU)] × (CS/CT) × 100
rU)] × (CS/CT) × 100
| rU | = | = peak response of orlistat related compound B from the Sample solution |
| rSP | = | = peak response of orlistat related compound B from the Spiked sample solution |
| CS | = | = concentration of USP Orlistat Related Compound B RS in the Standard solution (mg/mL) |
| CT | = | = concentration of Orlistat in the Spiked sample solution (mg/mL) |
Acceptance criteria:
NMT 0.05% of orlistat related compound B is found.
• Procedure 3
[Note—Avoid the use of plastic flasks for the preparation or containment of any solution in this analysis. ]
Mobile phase, Standard solution, and Sample solution:
Prepare as directed in the Assay.
System suitability solution:
10 µg/mL of USP Orlistat RS, 0.1 µg/mL of USP Orlistat Related Compound C RS, and 0.25 µg/mL of USP Orlistat Related Compound D RS in Mobile phase
Chromatographic system
Proceed as directed in the Assay, except to chromatograph the System suitability solution.
System suitability
Sample:
System suitability solution
Suitability requirements
Signal-to-noise ratio:
NLT 3 for the orlistat related compound C and orlistat related compound D peaks
Relative standard deviation:
NMT 10.0% for the orlistat peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response for each individual impurity from the Sample solution |
| rS | = | = peak response of USP Orlistat RS from the Standard solution |
| CS | = | = concentration of USP Orlistat RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Orlistat in the Sample solution (mg/mL) |
| F | = | = relative response factor as given in Impurity Table 1 |
Acceptance criteria:
See Impurity Table 1.
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Formylleucinea | 0.10 | 4.0 | 0.2 |
| Orlistat related compound C |
0.13 | 33 | 0.05 |
| Orlistat open ring epimerb | 0.44 | 1.0 | 0.2 |
| Orlistat related compound D* |
0.90 | — | Calculated in Procedure 4 |
| Orlistat open ring amidec* | 0.90 | — | Calculated in Procedure 4 |
| Orlistat | 1.00 | — | — |
| d-Leucine orlistatd | 1.18 | 1.0 | 0.2 |
| Individual unidentified impurity |
— | 1.0 | 0.1 |
|
*
Coelutes in this LC system, determined using Procedure 4.
a
N-Formyl-l-leucine.
b
(2S,3R,5S)-5-[(S)-2-Formylamino-4-methyl-pentanoyloxy]-2-hexyl-3-hydroxy-hexadecanoic acid.
c
N-Formyl-l-leucine (S)-1-[(2S,3S)-2-hydroxy-3-[1-phenyl-R-ethylcarbomoyl]nonyl]-dodecyl ester.
d
N-Formyl-d-leucine (S)-1-[[(2S,3S)-3-hexyl-4-oxo-2-
oxetanyl]methyl]dodecyl ester or enantiomer. |
|||
• Procedure 4: Limit of Orlistat Related Compound D
Mobile phase:
Methanol and water (83:17)
System suitability solution:
4 mg/mL of USP Orlistat RS and 2.4 µg/mL of USP Orlistat Related Compound D RS in acetonitrile, respectively
Standard solution:
5.0 mg/mL of USP Orlistat RS in acetonitrile
Sample solution:
5.0 mg/mL of Orlistat in acetonitrile
Chromatographic system
Mode:
LC
Detector:
205 nm
Column:
4.0-mm × 25-cm; 5-µm packing L7
Flow rate:
0.6 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Signal-to-noise ratio:
NLT 3 for the orlistat related compound D peak
Relative standard deviation:
NMT 10.0% for the orlistat peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response for each individual impurity from the Sample solution |
| rS | = | = peak response for USP Orlistat RS from the Standard solution |
| CS | = | = concentration of USP Orlistat RS in the Standard solution (µg/mL) |
| CU | = | = concentration of Orlistat in the Sample solution (µg/mL) |
| F | = | = relative response factor as obtained in Impurity Table 2 |
Acceptance criteria:
See Impurity Table 2.
Impurity Table 2
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Orlistat related compound D |
0.94 | 1.0 | 0.2 |
| Orlistat | 1.00 | — | — |
| Orlistat open ring amidea | 1.25 | 4.3 | 0.1 |
|
a
N-Formyl-l-leucine (S)-1-[(2S,3S)-2-hydroxy-3-[1-phenyl-R-ethylcarbomoyl]nonyl]-dodecyl ester.
|
|||
• Procedure 5: Limit of Orlistat Related Compound E
Buffer:
0.4 N borate solution, adjusted to a pH of 10.2
Derivitizing agent:
o-Phthaldehyde (OPA) solution. [Note—If unable to obtain commercially, the Derivitizing agent can be prepared as 1% each of 3-mercaptopropionic acid and o-phthaldialdehyde in 0.4 M borate buffer solution. ]
Solution A:
Transfer 4.1 g of sodium acetate trihydrate and 40 mg of ethylenediaminetetraacetic acid (EDTA) into a 1-L volumetric flask. Dissolve in 950 mL of water, and adjust with 0.1 N sodium hydroxide to a pH of 7.2. Dilute with water to volume, add 2.5 mL of tetrahydrofuran, and mix. Filter, and degas.
Solution B:
Transfer 2.7 g of sodium acetate trihydrate and 40 mg of EDTA into a 1-L volumetric flask. Dissolve in 200 mL of water, and adjust with 0.1 N sodium hydroxide to a pH of 7.2. Add 800 mL of acetonitrile, filter, and degas.
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 96.7 | 3.3 |
| 20 | 60 | 40 |
| 24 | 0 | 100 |
| 38 | 0 | 100 |
| 38 | 96.7 | 3.3 |
| 45 | 96.7 | 3.3 |
Standard solution:
Transfer a weighed quantity of about 0.2 mg of USP Orlistat Related compound E RS into a 20-mL head-space vial. Add 10 mL of 4 N sodium hydroxide, and close the vial. Heat the vial to 100 for 1 h, then allow to cool to room temperature. Transfer 2 mL of the resulting solution into a 50-mL volumetric flask, and dilute with water to volume. To 0.5 mL of this solution add 2.0 mL of Buffer and 0.5 mL of Derivatizing agent.
for 1 h, then allow to cool to room temperature. Transfer 2 mL of the resulting solution into a 50-mL volumetric flask, and dilute with water to volume. To 0.5 mL of this solution add 2.0 mL of Buffer and 0.5 mL of Derivatizing agent.
Sample solution:
Proceed as directed for the Standard solution, but instead use 25 mg of Orlistat to replace the 0.2 mg of USP Orlistat Related Compound E RS.
Chromatographic system
Mode:
LC
Detector:
Fluorescence 340 nm (excitation); 450 nm (emission)
Columns
Guard:
2.1-mm × 2-cm; 50-µm packing L1
Analytical:
2.1-mm × 20-cm; packing L1
Flow rate:
0.5 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 6.0% for the orlistat related compound E peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of this impurity in the portion of Orlistat taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response for orlistat related compound E in the Sample solution |
| rS | = | = peak response for USP Orlistat Related Compound E RS in the Standard solution |
| CS | = | = concentration of USP Orlistat Related Compound E RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Orlistat in the Sample solution (mg/mL) |
Acceptance criteria
Individual impurity:
NMT 0.2% of orlistat related compound E is found.
Total impurities:
NMT 1.0% of total impurities is found, the results for Procedures 1, 2, 3, 4, and 5 being added.
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781
781
Sample solution:
30 mg/mL in dehydrated alcohol
Acceptance criteria:
Between  48.0
48.0 and
and  51.0
51.0 , at 20
, at 20
• Water Determination, Method Ic  921
921 :
NMT 0.2%
:
NMT 0.2%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers between 2 and 8
and 8 .
.
• USP Reference Standards  11
11
USP Orlistat RS
USP Orlistat Related Compound A RS
USP Orlistat Related Compound B RS
USP Orlistat Related Compound C RS
USP Orlistat Related Compound D RS
USP Orlistat Related Compound E RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 4130
Pharmacopeial Forum: Volume No. 35(5) Page 1166