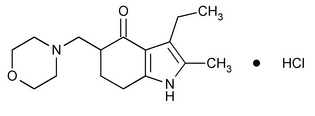
Molindone Hydrochloride
(moe lin' done hye'' droe klor' ide).
4H-Indol-4-one, 3-ethyl-1,5,6,7-tetrahydro-2-methyl-5-(4-morpholinylmethyl)-, monohydrochloride.
3-Ethyl-6,7-dihydro-2-methyl-5-(morpholinomethyl)indol-4(5H)-one monohydrochloride
» Molindone Hydrochloride contains not less than 98.0 percent and not more than 101.5 percent of C16H24N2O2·HCl, calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight, light-resistant containers.
Identification—
A:
Infrared Absorption  197K
197K . Do not dry specimens.
. Do not dry specimens.
B:
Prepare a solution in methanol containing 10 mg of molindone hydrochloride per mL. Separately apply 1 µL of this solution and 1 µL of a Standard solution containing 10 mg per mL of USP Molindone Hydrochloride RS in methanol to a thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture, and allow the spots to dry. Protect the chromatogram from light, and develop in a solvent system consisting of a mixture of alcohol, methanol, and 1 N hydrochloric acid (90:5:5) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate. Spray the plate with a freshly prepared solution containing 100 mg of potassium ferricyanide dissolved in 20 mL of 10% ferric chloride solution: the principal spot obtained from the test solution corresponds in RF value and intensity to that obtained from the Standard solution.
) coated with a 0.25-mm layer of chromatographic silica gel mixture, and allow the spots to dry. Protect the chromatogram from light, and develop in a solvent system consisting of a mixture of alcohol, methanol, and 1 N hydrochloric acid (90:5:5) until the solvent front has moved about three-fourths of the length of the plate. Remove the plate from the developing chamber, mark the solvent front, and allow the solvent to evaporate. Spray the plate with a freshly prepared solution containing 100 mg of potassium ferricyanide dissolved in 20 mL of 10% ferric chloride solution: the principal spot obtained from the test solution corresponds in RF value and intensity to that obtained from the Standard solution.
C:
It responds to the tests for Chloride  191
191 .
.
pH  791
791 :
between 4.0 and 5.0, in a solution (1 in 100).
:
between 4.0 and 5.0, in a solution (1 in 100).
Water, Method I  921
921 :
not more than 0.5%.
:
not more than 0.5%.
Residue on ignition  281
281 :
not more than 0.25%.
:
not more than 0.25%.
Heavy metals, Method II  231
231 :
not more than 0.003%.
:
not more than 0.003%.
Chromatographic purity—
Mobile phase—
Dissolve 1.1 g of sodium octanesulfonate in 600 mL of water, add 400 mL of methanol, 1 mL of glacial acetic acid, and 0.5 mL of triethylamine. Mix, filter through a filter having a porosity of 0.45 µm or less, and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Solvent mixture—
Proceed as directed in the Assay.
Standard solution—
Prepare a solution of USP Molindone Hydrochloride RS in Solvent mixture having a known concentration of about 0.01 mg per mL.
Test solution—
Transfer about 100 mg of Molindone Hydrochloride, accurately weighed, to a 50-mL volumetric flask, dissolve in and dilute with Solvent mixture to volume.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L11. The column temperature is maintained at 35
)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L11. The column temperature is maintained at 35 . The flow rate is about 1.5 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed under Procedure: the relative standard deviation for replicate injections is not more than 5.0%.
. The flow rate is about 1.5 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed under Procedure: the relative standard deviation for replicate injections is not more than 5.0%.
Procedure—
Separately inject equal volumes (about 20 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the responses of all peaks: no peak from the Test solution, other than the molindone peak, is greater than the molindone peak from the Standard preparation (0.5%), and the sum of all the impurity peaks is not greater than 2.0%.
Assay—
Mobile phase—
Dissolve 1.1 g of sodium octanesulfonate in 480 mL of water, add 520 mL of methanol, 2 mL of glacial acetic acid, and 0.4 mL of triethylamine. Mix, filter through a 0.45-µm filter, and degas. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Solvent mixture—
Prepare a mixture of 0.01 N hydrochloric acid and methanol (60:40).
Internal standard solution—
Dissolve 200 mg of butylparaben in 40 mL of methanol in a 100-mL volumetric flask, dilute with water to volume, and mix.
Standard preparation—
Transfer about 25 mg of USP Molindone Hydrochloride RS, accurately weighed, to a 50-mL volumetric flask, add 5.0 mL of Internal standard solution, dilute with Solvent mixture to volume, and mix.
Assay preparation—
Transfer about 50 mg of Molindone Hydrochloride, accurately weighed, to a 100-mL volumetric flask. Add 10.0 mL of Internal standard solution, dilute with Solvent mixture to volume, and mix.
Chromatographic system
(see Chromatography  621
621 )—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L11. The column temperature is maintained at 35
)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm × 25-cm column that contains packing L11. The column temperature is maintained at 35 . The flow rate is 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the resolution, R, between the molindone and butylparaben peaks is not less than 2, and the relative standard deviation for replicate injections is not more than 2.0%.
. The flow rate is 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed under Procedure: the resolution, R, between the molindone and butylparaben peaks is not less than 2, and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the responses for the major peaks. The relative retention times are about 0.7 for molindone and 1.0 for butylparaben. Calculate the quantity, in mg, of C16H24N2O2·HCl in the portion of Molindone Hydrochloride taken by the formula:
100C(RU / RS)
in which C is the concentration, in mg per mL, of USP Molindone Hydrochloride RS in the Standard preparation, and RU and RS are the ratios of the peak response of molindone to that of butylparaben obtained from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3942