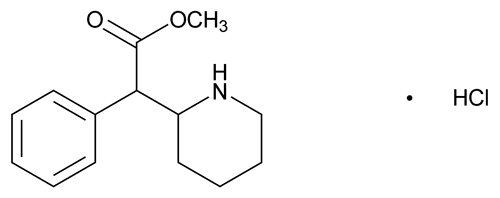
Methylphenidate Hydrochloride
C14H19NO2·HCl 269.77
2-Piperidineacetic acid, -phenyl-, methyl ester, hydrochloride, (R*,R*)-(±)-;
-phenyl-, methyl ester, hydrochloride, (R*,R*)-(±)-;
Methyl -phenyl-2-piperidineacetate hydrochloride
-phenyl-2-piperidineacetate hydrochloride


 [298-59-9].
[298-59-9].
(meth'' il fen' i date hye'' droe klor' ide).
C14H19NO2·HCl 269.77
2-Piperidineacetic acid,
Methyl
DEFINITION
Methylphenidate Hydrochloride contains NLT 98.0% and NMT 102.0% of C14H19NO2·HCl, calculated on the dried basis.
IDENTIFICATION
• B. Identification Tests—General, Chloride  191
191 :
Meets the requirement for the silver nitrate precipitate test for chloride
:
Meets the requirement for the silver nitrate precipitate test for chloride
ASSAY
• Procedure
Buffer:
2.7 g/L of monobasic potassium phosphate
Mobile phase:
Methanol and Buffer (1:2). Adjust with phosphoric acid to a pH of 4.6 ± 0.1.
System suitability solution:
0.005 mg/mL of USP Methylphenidate Related Compound A RS and 0.5 mg/mL of USP Methylphenidate Hydrochloride RS in Mobile phase
Standard solution:
0.5 mg/mL of USP Methylphenidate Hydrochloride RS in Mobile phase
Sample solution:
0.5 mg/mL of Methylphenidate Hydrochloride in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 209 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1.0 mL/min
Injection size:
10 µL
Run Time:
2 times the retention time of methylphenidate
System suitability
Sample:
System suitability solution
Suitability requirements
Tailing factor:
NMT 3.0 for the methylphenidate peak
Resolution:
NLT 2.5 between methylphenidate related compound A and methylphenidate
Relative standard deviation:
NMT 2.0% for the methylphenidate peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C14H19NO2·HCl in the portion taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Methylphenidate Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0, calculated on the dried basis
IMPURITIES
Organic Impurities
[Note—If ethylphenidate or bis-methylphenidate is a known process impurity, Procedure 2 is recommended. ]
• Procedure 1
Buffer, Mobile phase, System suitability solution, Sample solution, Chromatographic system, and System suitability:
Proceed as directed in the Assay.
Analysis
Sample:
Sample solution
Identify each impurity using the relative retention times in Impurity Table 1. Calculate the percentage of each impurity in the portion of Methylphenidate Hydrochloride taken:
Result = (rU/rT) × 100
| rU | = | = peak response for each impurity in the Sample solution |
| rT | = | = sum of the responses for all impurity peaks including the methylphenidate peak in the Sample solution |
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 1.0%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Erythro(R,S) isomera | 0.58 | 0.15 |
| Methylphenidate related compound Ab | 0.85 | 0.5 |
| Methylphenidate | 1.0 | — |
| Any individual, unspecified impurity | — | 0.10 |
|
a
Methyl (RS,SR)-2-phenyl-2-(piperidin-2-yl)acetate.
b
(RS,RS)2-Phenyl-2-(piperidin-2-yl)acetic acid. [Note—Also known as
|
||
• Procedure 2
[Note—Perform this test only if ethylphenidate or bis-1,2-(-carboxymethylbenzyl) piperidine is a known process impurity. ]
Buffer A:
5.7 g of monobasic ammonium phosphate and 1.6 g of 1-octanesulfonate sodium in 1 L of water
Buffer B:
Add 4 mL of triethylamine to 1 L of Buffer A. Adjust with phosphoric acid to a pH of 2.9.
Solution A:
Acetonitrile and Buffer B (7:43)
Solution B:
Acetonitrile and Buffer A (4:1)
Standard solution:
0.5 µg/mL of USP Methylphenidate Hydrochloride RS in Solution A
System suitability solution:
0.5 mg/mL of USP Methylphenidate Hydrochloride RS; and 3 µg/mL each of USP Methylphenidate Related Compound A RS, phenylacetic acid, and USP Methylphenidate Hydrochloride Erythro Isomer Solution RS in Solution A
Sample solution:
0.5 mg/mL of Methylphenidate Hydrochloride in Solution A. [Note—Allow the solution to stand for at least 2 h. ]
Mobile phase:
See the gradient table below. (See also Chromatography  621
621 , System Suitability).
, System Suitability).
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 90 | 10 |
| 7 | 65 | 35 |
| 10 | 50 | 50 |
| 12 | 50 | 50 |
| 13 | 90 | 10 |
| 16 | 90 | 10 |
[Note—Equilibration of the chromatographic system at the initial conditions for a minimum of 30 min is recommended before the first injection. ]
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
3.9-mm × 15-cm; 5-µm packing L7
Column temperature:
40
Flow rate:
2.8 mL/min
Injection size:
10 µL
System suitability
Sample:
System suitability solution. [Note—Identify the peaks using the relative retention times in Impurity Table 2. ]
Suitability requirements
Resolution:
NLT 2.7 between methylphenidate related compound A and phenylacetic acid; NLT than 3.6 between phenylacetic acid and erythro isomer
Tailing factor:
NMT 2.0 for the methylphenidate peak
Relative standard deviation:
NMT 2.0% for the methylphenidate peak; NMT 5.0% for methylphenidate related compound A, phenylacetic acid, and methylphenidate hydrochloride erythro isomer
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of any individual impurity in the portion of Methylphenidate Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response for each impurity peak from the Sample solution |
| rS | = | = peak response for the methylphenidate peak from the Standard solution |
| CS | = | = concentration of USP Methylphenidate Hydrochloride RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Methylphenidate Hydrochloride in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Impurity Table 2) |
Impurity acceptance criteria
Individual impurities:
See Impurity Table 2.
Total impurities:
NMT 0.5%
Impurity Table 2
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Methylphenidate related compound Aa | 0.55 | 1.1 | 0.2 |
| Phenylacetic acid | 0.67 | 1.0 | 0.1 |
| Erythro(R,S) isomerb | 0.80 | 1.0 | 0.2 |
| Methylphenidate | 1.0 | — | — |
| Ethylphenidatec | 1.22 | 0.9 | 0.1 |
| Bis-methylphenidated | 1.80 | 2.6 | 0.1 |
| Any individual, unspecified impurity | — | 1.0 | 0.1 |
|
a
(RS,SR)2-Phenyl-2-(piperidin-2-yl)acetic acid. [Note—Also known as
b
Methyl (RS,SR)-2-phenyl-2-(piperidin-2-yl)acetate.
c
Ethyl (RR,SS)-2-phenyl-2-(piperidin-2-yl)acetate.
d
1,2-Bis(carboxymethylbenzyl)piperidine. [Note—Also known as 1,2-(
|
|||
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample in a vacuum at 60
:
Dry a sample in a vacuum at 60 for 4 h: it loses NMT 0.5% of its weight.
for 4 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers.
• Labeling
If a test for Organic Impurities other than Procedure 1 is used, then the labeling states the procedure with which the article complies.
• USP Reference Standards  11
11
USP Methylphenidate Hydrochloride Erythro Isomer Solution RS
This solution contains 0.5 mg of methylphenidate hydrochloride erythro isomer per mL in methanol.
This solution contains 0.5 mg of methylphenidate hydrochloride erythro isomer per mL in methanol.
USP Methylphenidate Related Compound A RS
 -Phenyl-2-piperidineacetic acid hydrochloride.
-Phenyl-2-piperidineacetic acid hydrochloride.
C13H17NO2·HCl 255.75
C13H17NO2·HCl 255.75
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3880
Pharmacopeial Forum: Volume No. 36(1) Page 119