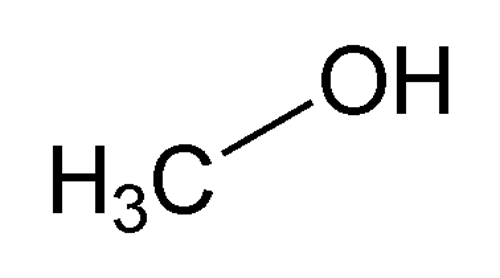
Methyl Alcohol
(meth' il al' ka hol).
DEFINITION
Methyl Alcohol contains NLT 99.5% of CH3OH.
[Caution—Methyl Alcohol is poisonous.
]
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
System suitability solution:
Dilute 1.0 mL of USP Methyl Alcohol RS and 1.0 mL of USP Acetone RS with tetrahydrofuran to 50 mL.
Internal standard solution:
2% (v/v) acetonitrile in tetrahydrofuran
Standard solution:
15.8 mg/mL of USP Methyl Alcohol RS in Internal standard solution
Sample solution:
15.8 mg/mL of Methyl Alcohol in Internal standard solution
Chromatographic system
Detector:
Flame ionization
Column:
0.32-mm × 30-m fused-silica capillary column, coated with a 1.8-µm layer of phase G43
Temperature
Injector:
200
Detector:
280
Column:
See Table 1.
Table 1
| Initial Temperature ( |
Temperature Ramp ( |
Final Temperature ( |
Hold Time at Final Temperature (min) |
|---|---|---|---|
| 40 | — | 40 | 5 |
| 40 | 20 | 240 | — |
Carrier gas:
Helium
Linear velocity:
35 cm/s
Injection type:
Split ratio, 20:1
Injection size:
1 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for methyl alcohol, acetone, and acetonitrile are 1.0, about 1.6, and about 1.8, respectively. ]
Suitability requirements
Resolution:
NLT 15 between methyl alcohol and acetone, System suitability solution
Tailing factor:
NLT 1.5 for methyl alcohol, System suitability solution
Relative standard deviation:
NMT 2.0% for the ratio of the peak area of methyl alcohol to acetonitrile, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of methyl alcohol (CH3OH) in the portion of Methyl Alcohol taken:
Result = (RU/RS) × (CS/CU) × 100
| RU | = | = peak area ratio from the Sample solution |
| RS | = | = peak area ratio from the Standard solution |
| CS | = | = concentration of USP Methyl Alcohol RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Methyl Alcohol in the Sample solution (mg/mL) |
Acceptance criteria:
NLT 99.5%
IMPURITIES
• Nonvolatile Residue
Sample:
250 mL of Methyl Alcohol
Analysis:
Evaporate the Sample in a 600-mL beaker on a steam bath, in a well-ventilated hood, until the volume is reduced to about 100 mL. Cool, transfer a portion of the liquid to a suitable, tared 50-mL platinum dish on a steam bath, and evaporate. Repeat the process until all of the liquid has been transferred, and then evaporate to dryness. Dry at 105 for 30 min, cool, and weigh.
for 30 min, cool, and weigh.
Acceptance criteria:
The weight of the residue does not exceed 2 mg, corresponding to NMT 0.001% (w/w).
• Acetone and Aldehydes (as acetone)
Standard solution:
Dilute 1.9 mL (1.5 g) of acetone with water to 1000 mL, then dilute 1.0 mL of this solution with water to 100 mL. Dilute 2 mL of the resulting solution with water to 5 mL. The Standard solution contains 30 µg of acetone and is freshly prepared.
Sample solution:
Dilute 1.25 mL (1 g) of Methyl Alcohol with water to 5 mL.
Analysis:
Adjust to and maintain each solution at 20 . Add 5 mL of alkaline mercuric–potassium iodide TS to each of the Standard solution and Sample solution.
. Add 5 mL of alkaline mercuric–potassium iodide TS to each of the Standard solution and Sample solution.
Acceptance criteria:
Any turbidity produced in the Sample solution is not greater than that produced in the Standard solution (NMT 0.003%).
• Readily Carbonizable Substances  271
271
Sample:
5 mL
Analysis:
Cool 5 mL of sulfuric acid, contained in a small conical flask, to 10 , and add the Sample dropwise with constant mixing, maintaining the temperature below 20
, and add the Sample dropwise with constant mixing, maintaining the temperature below 20 throughout the test.
throughout the test.
Acceptance criteria:
No discoloration develops.
• Readily Oxidizable Substances
Sample:
20 mL of Methyl Alcohol
Analysis:
Cool the Sample to 15 , add 0.1 mL of 0.1 N potassium permanganate, and allow to stand at 15
, add 0.1 mL of 0.1 N potassium permanganate, and allow to stand at 15 .
.
Acceptance criteria:
The pink color does not completely disappear within 5 min.
SPECIFIC TESTS
• Acidity
Sample solution:
Mix 25 mL of water with 10 mL of alcohol and 0.5 mL of phenolphthalein TS, and add 0.02 N sodium hydroxide until a slight pink color persists after shaking for 30 s. Taking precautions to avoid absorption of carbon dioxide, add 19 mL (15 g) of Methyl Alcohol.
Analysis:
Titrate the Sample solution with 0.020 N sodium hydroxide.
Acceptance criteria:
NMT 0.45 mL of 0.020 N sodium hydroxide is required to produce a pink color.
• Alkalinity (as ammonia)
Sample:
28.6 mL (22.6 g) of Methyl Alcohol
Analysis:
Mix the Sample with 25 mL of water, add 1 drop of methyl red TS, and titrate with 0.020 N sulfuric acid.
Acceptance criteria:
NMT 0.20 mL of 0.020 N sulfuric acid is required to produce a pink color (3 ppm).
• Water Determination, Method I  921
921 :
NMT 0.1%
:
NMT 0.1%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, remote from heat, sparks, and open flames.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1865
Pharmacopeial Forum: Volume No. 34(5) Page 1226