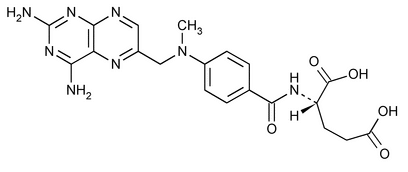
Methotrexate
C20H22N8O5 454.44
l-Glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-;
l-(+)-N-[p-[[(2,4-Diamino-6-pteridinyl)methyl]methylamino]benzoyl] glutamic acid;
(S)-2-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)pentanedioic acid

 [59-05-2].
[59-05-2].
(meth'' oh trex' ate).
C20H22N8O5 454.44
l-Glutamic acid, N-[4-[[(2,4-diamino-6-pteridinyl)methyl]methylamino]benzoyl]-;
l-(+)-N-[p-[[(2,4-Diamino-6-pteridinyl)methyl]methylamino]benzoyl] glutamic acid;
(S)-2-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)pentanedioic acid
DEFINITION
Methotrexate is a mixture of 4-amino-10-methylfolic acid and closely related compounds. It contains NLT 98.0% and NMT 102.0% of C20H22N8O5, calculated on the anhydrous basis.
[Caution—Great care should be taken to prevent inhaling particles of Methotrexate and exposing the skin to it.
]
IDENTIFICATION
• A. Infrared Absorption  197K
197K :
Do not dry specimens.
:
Do not dry specimens.
• B. Ultraviolet Absorption  197U
197U
Sample solution:
10 µg/mL in 0.1 N hydrochloric acid
ASSAY
• Procedure
Buffer:
3.4 mg/mL of anhydrous monobasic sodium phosphate in water. Adjust with 1 N sodium hydroxide to a pH of 6.0.
Solution A:
Acetonitrile and Buffer (1:19)
Solution B:
Acetonitrile and Buffer (1:1)
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 30 | 50 | 50 |
| 34 | 50 | 50 |
| 35 | 100 | 0 |
| 40 | 100 | 0 |
Standard stock solution:
1.0 mg/mL of USP Methotrexate RS prepared as follows. Transfer a known amount of USP Methotrexate RS to a suitable volumetric flask, dissolve in dimethyl sulfoxide equivalent to 5% of the final volume, and dilute with Solution A to volume.
Standard solution:
0.2 mg/mL of USP Methotrexate RS in Solution A, from the Standard stock solution
Sample stock solution:
Transfer 200 mg of Methotrexate to a 200-mL volumetric flask, and dissolve in 10 mL of dimethyl sulfoxide with sonication for 5 min. Add 150 mL of Solution A, and sonicate again for 5 min. Dilute with Solution A to volume to obtain 1.0 mg/mL of Methotrexate. [Note—Sonicate as needed. ]
Sample solution:
0.2 mg/mL of Methotrexate in Solution A, from the Sample stock solution
Chromatographic system
Mode:
LC
Detector:
UV 280 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1.0 mL/min
Injection size:
20 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 1.6
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C20H22N8O5 in the portion of Methotrexate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Methotrexate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Methotrexate in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Organic Impurities
• Procedure 1: Related Compounds
Solution A, Solution B, Mobile phase, Sample solution, and Chromatographic system:
Proceed as directed in the Assay.
Standard stock solution A:
Use the Standard solution from the Assay.
Standard solution A:
0.1 µg/mL of USP Methotrexate RS in Solution A, from the Standard stock solution
Standard stock solution B:
Transfer known quantities of USP Methotrexate Related Compound C RS, USP Methotrexate Related Compound B RS, and USP Methotrexate Related Compound E RS to a suitable volumetric flask, dissolve in dimethyl sulfoxide equivalent to 1% of the final volume, and dilute with Solution A to volume to obtain 0.1 mg/mL of USP Methotrexate Related Compound B RS, 0.2 mg/mL of USP Methotrexate Related Compound C RS, and 0.1 mg/mL of USP Methotrexate Related Compound E RS.
Standard solution B:
0.4 µg/mL of USP Methotrexate Related Compound C RS, 0.2 µg/mL of USP Methotrexate Related Compound B RS, and 0.2 µg/mL of USP Methotrexate Related Compound E RS in Solution A, from Standard stock solution B
System suitability solution:
Transfer a known quantity of USP Methotrexate System Suitability Mixture RS to a suitable volumetric flask, and dissolve in dimethyl sulfoxide equivalent to about 1% of the final volume. Add Standard stock solution B equivalent to 0.2% of the final volume, and dilute with Solution A to volume to prepare 0.1 mg/mL of USP Methotrexate System Suitability Mixture RS, 0.2 µg/mL of USP Methotrexate Related Compound B RS, 0.4 µg/mL of USP Methotrexate Related Compound C RS, and 0.2 µg/mL of USP Methotrexate Related Compound E RS.
System suitability
Samples:
Standard solution A and System suitability solution
Suitability requirements
Resolution:
NLT 1.7 between methotrexate related compound B and methotrexate related compound C, NLT 10.0 between methotrexate related compound C and methotrexate, and NLT 5.0 between methotrexate related compound I and methotrexate related compound H; System suitability solution
Relative standard deviation:
NMT 5.0%, Standard solution A
Analysis
[Note—USP Methotrexate Related Compound E RS is 4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}benzoic acid, hemihydrochloride. ]
Samples:
Standard solution A, Standard solution B, and Sample solution
Calculate the percentage of methotrexate related compound B and methotrexate related compound C in the portion of Methotrexate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rs | = | = peak response from Standard solution B |
| CS | = | = concentration of the corresponding methotrexate related compound in Standard solution B (mg/mL) |
| CU | = | = concentration of Methotrexate in the Sample solution (mg/mL) |
Calculate the percentage of methotrexate related compound E free acid in the portion of Methotrexate taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from Standard solution B |
| CS | = | = concentration of USP Methotrexate Related Compound E RS in Standard solution B (mg/mL) |
| CU | = | = concentration of Methotrexate in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of methotrexate related compound E free acid, 325.33 |
| Mr2 | = | = molecular weight of USP Methotrexate Related Compound E RS, 343.56 |
Calculate the percentage of methotrexate related compound H, methotrexate related compound I, and any unspecified impurity in the portion of Methotrexate taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response from the Sample solution |
| rs | = | = peak response of methotrexate from Standard solution A |
| CS | = | = concentration of USP Methotrexate RS in Standard solution A (mg/mL) |
| CU | = | = concentration of Methotrexate in the Sample solution (mg/mL) |
| F | = | = relative response factor for each individual impurity (see Impurity Table 1) |
Acceptance criteria
Individual impurities:
See Impurity Table 1. [Note—Disregard any impurity peak less than 0.05%. ]
Total impurities:
NMT 1.0%
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Methotrexate related compound Ba | 0.71 | — | 0.3 |
| Methotrexate related compound Cb | 0.75 | — | 0.5 |
| Methotrexate | 1.00 | — | — |
| Methotrexate related compound E free acidc | 1.39 | — | 0.3 |
| Methotrexate dimethylamided and Methotrexate related compound Ie | 1.55 | 0.71 | 0.2f |
| Methotrexate related compound Hg | 1.68 | 1.0 | 0.2 |
| Any unspecified impurity | — | 1.0 | 0.10 |
|
a
(S)-2-{4-[(2,4-Diaminopteridin-6-yl)methylamino]benzamido}pentanedioic acid.
b
(S)-2-{4-[(2-Amino-4-oxo-1,4-dihydropteridin-6-yl)methylamino]-N-methylbenzamido}pentanedioic acid.
c
4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzoic acid.
d
2-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)-5-(dimethylamino)-5-oxopentanoic acid.
e
(S)-4-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)-5-methoxy-5-oxopentanoic acid.
f
If present, methotrexate dimethylamide and Methotrexate related compound I may not be completely resolved by the method. These peaks are integrated together to determine conformance.
g
(S)-2-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)-5-methoxy-5-oxopentanoic acid.
|
|||
• Procedure 2: Enantiomeric Purity
Solution A:
7.1 g/L of anhydrous dibasic sodium phosphate in water
Solution B:
6.9 g/L of monobasic sodium phosphate in water
Solution C:
Solution A and Solution B (5:6). Adjust with 2 N sodium hydroxide to a pH of 6.9.
Mobile phase:
n-Propanol and Solution C (2:23)
System suitability solution:
0.02 mg/mL each of USP Methotrexate RS and USP R-Methotrexate RS in Mobile phase
Sample solution:
0.2 mg/mL of Methotrexate in Mobile phase
Diluted sample solution:
2 µg/mL of Methotrexate in Mobile phase, from the Sample solution
Chromatographic system
Mode:
LC
Detector:
UV 302 nm
Column:
4.0-mm × 15-cm; 7-µm packing L75
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution. [Note—The relative retention times for methotrexate and R-methotrexate are 1.0 and 1.95, respectively. ]
Suitability requirements
Resolution:
NLT 1.3 between methotrexate and R-methotrexate
Relative standard deviation:
NMT 5.0% for the methotrexate peak
Analysis
Samples:
Sample solution and Diluted sample solution
Calculate the percentage of R-methotrexate in the portion of Methotrexate taken:
Result = [rU/(rS × 100)] × 100
| rU | = | = peak area of R-methotrexate from the Sample solution |
| rS | = | = peak area of Methotrexate from the Diluted sample solution |
Acceptance criteria:
NMT 3.0%
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
NMT 12.0%
:
NMT 12.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers.
• USP Reference Standards  11
11
(S)-4-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)-5-methoxy-5-oxopentanoic acid.
C21H24N8O5 468.47
(S)-2-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)-5-methoxy-5-oxopentanoic acid.
C21H24N8O5 468.47
USP Methotrexate Related Compound B RS
USP Methotrexate Related Compound C RS
USP Methotrexate Related Compound E RS
USP Methotrexate System Suitability Mixture RS
It contains Methotrexate, Methotrexate Dimethylester Hydrochloride
(S)-Dimethyl-2-(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}benzamido) pentanedioate hydrochloride.
C22H26N8O5·HCl 518.95
It contains Methotrexate, Methotrexate Dimethylester Hydrochloride
(S)-Dimethyl-2-(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)amino}benzamido) pentanedioate hydrochloride.
C22H26N8O5·HCl 518.95
and a small amount of Methotrexate related compound I
(S)-4-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)-5-methoxy-5-oxopentanoic acid.
C21H24N8O5 468.47
and Methotrexate related compound H
(S)-2-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)-5-methoxy-5-oxopentanoic acid.
C21H24N8O5 468.47
USP R-Methotrexate RS
(R)-2-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)pentanedioic acid.
C20H22N8O5 454.44
(R)-2-(4-{[(2,4-Diaminopteridin-6-yl)methyl](methyl)amino}benzamido)pentanedioic acid.
C20H22N8O5 454.44
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Feiwen Mao, M.S.
Senior Scientific Liaison 1-301-816-8320 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3855
Pharmacopeial Forum: Volume No. 36(4) Page 925