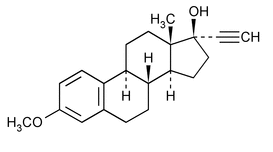
Mestranol
(mes' tra nol).
19-Norpregna-1,3,5(10)-trien-20-yn-17-ol, 3-methoxy-, (17
3-Methoxy-19-nor-17
» Mestranol contains not less than 97.0 percent and not more than 102.0 percent of C21H26O2, calculated on the dried basis.
Packaging and storage—
Preserve in well-closed, light-resistant containers.
Identification—
Solution:
100 µg per mL.
Medium:
methanol.
C:
Prepare a solution in chloroform to contain 1 mg of mestranol per mL. Apply 10 µL of this solution and 10 µL of a solution of USP Mestranol RS in chloroform containing 1 mg per mL to a line parallel to and about 2.5 cm from the bottom edge of a thin-layer chromatographic plate (see Chromatography  621
621 ) coated with a 0.25-mm layer of chromatographic silica gel mixture. Place the plate in a developing chamber containing and equilibrated with a mixture of 29 volumes of chloroform and 1 volume of dehydrated alcohol. Develop the chromatogram until the solvent front has moved about 15 cm above the line of application. Remove the plate, allow the solvent to evaporate, and spray with Methanol–sulfuric acid prepared as described in the Assay. Heat the plate in an oven at 105
) coated with a 0.25-mm layer of chromatographic silica gel mixture. Place the plate in a developing chamber containing and equilibrated with a mixture of 29 volumes of chloroform and 1 volume of dehydrated alcohol. Develop the chromatogram until the solvent front has moved about 15 cm above the line of application. Remove the plate, allow the solvent to evaporate, and spray with Methanol–sulfuric acid prepared as described in the Assay. Heat the plate in an oven at 105 for 5 minutes, and observe under long-wavelength UV light: the RF value of the principal spot obtained from the solution under test corresponds to that obtained with the Standard solution.
for 5 minutes, and observe under long-wavelength UV light: the RF value of the principal spot obtained from the solution under test corresponds to that obtained with the Standard solution.
Melting range  741
741 :
between 146
:
between 146 and 154
and 154 , but the range between beginning and end of melting does not exceed 4
, but the range between beginning and end of melting does not exceed 4 .
.
Specific rotation  781S
781S :
between +2
:
between +2 and +8
and +8 .
.
Test solution:
20 mg, previously dried, per mL, in dioxane.
Loss on drying  731
731 —
Dry it at 105
—
Dry it at 105 for 3 hours: it loses not more than 1.0% of its weight.
for 3 hours: it loses not more than 1.0% of its weight.
Assay—
Methanol–sulfuric acid—
Pipet 30 mL of methanol into a 100-mL volumetric flask contained in an ice bath. Add slowly and cautiously, and with continuous stirring, about 65 mL of sulfuric acid, taking care that the temperature remains below 15 . Allow the solution to warm to room temperature, and dilute with sulfuric acid to 100 mL.
. Allow the solution to warm to room temperature, and dilute with sulfuric acid to 100 mL.
Standard preparation—
Dissolve a suitable quantity of USP Mestranol RS, accurately weighed, in chloroform, and dilute quantitatively and stepwise with chloroform to obtain a solution having a known concentration of about 5 µg per mL.
Assay preparation—
Weigh accurately about 20 mg of Mestranol, dissolve in chloroform to make 200.0 mL, and mix. Pipet 5 mL of this solution into a 100-mL volumetric flask, add chloroform to volume, and mix.
Procedure—
Pipet 4 mL each of the Standard preparation and the Assay preparation into separate glass-stoppered, 25-mL conical flasks. Evaporate the solutions under a slow stream of air, without the aid of heat, to dryness. Dissolve the residue in 0.3 mL of methanol. Place the flasks in a water bath maintained at a temperature of 25 , and pipet into each, with constant swirling, 10 mL of Methanol–sulfuric acid. Insert the stoppers in the flasks. At 6 minutes, accurately timed, after the addition of the Methanol–sulfuric acid, concomitantly determine the absorbances of the solutions obtained from the Assay preparation and the Standard preparation at the wavelength of maximum absorbance at about 545 nm, with a suitable spectrophotometer, using Methanol–sulfuric acid as the blank. Calculate the quantity, in mg, of C21H26O2 in the Mestranol taken by the formula:
, and pipet into each, with constant swirling, 10 mL of Methanol–sulfuric acid. Insert the stoppers in the flasks. At 6 minutes, accurately timed, after the addition of the Methanol–sulfuric acid, concomitantly determine the absorbances of the solutions obtained from the Assay preparation and the Standard preparation at the wavelength of maximum absorbance at about 545 nm, with a suitable spectrophotometer, using Methanol–sulfuric acid as the blank. Calculate the quantity, in mg, of C21H26O2 in the Mestranol taken by the formula:
4C(AU / AS)
in which C is the concentration, in µg per mL, of USP Mestranol RS in the Standard preparation; and AU and AS are the absorbances of the solutions from the Assay preparation and the Standard preparation, respectively.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Mary S. Waddell
Scientific Liaison 1-301-816-8124 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3824