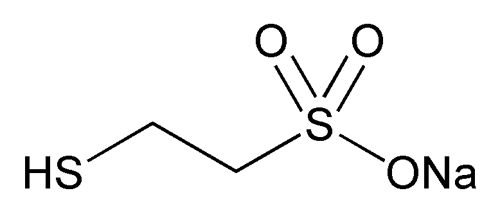
Mesna
C2H5NaO3S2 164.18
Ethanesulfonic acid, 2-mercapto-, monosodium salt;
Sodium 2-mercaptoethanesulfonate;
Sodium 2-sulphanylethanesulfonate

 [19767-45-4].
[19767-45-4].
(mes' na).
C2H5NaO3S2 164.18
Ethanesulfonic acid, 2-mercapto-, monosodium salt;
Sodium 2-mercaptoethanesulfonate;
Sodium 2-sulphanylethanesulfonate
DEFINITION
Mesna contains NLT 96.0% and NMT 102.0% of C2H5NaO3S2, calculated on the dried basis.
IDENTIFICATION
• B. Identification Tests—General, Sodium  191
191 :
A solution meets the requirements of the flame test.
:
A solution meets the requirements of the flame test.
ASSAY
• Procedure
Sample solution:
120 mg of Mesna in 10 mL of water
Analysis:
To the Sample solution add 10 mL of 1 M sulfuric acid and 10 mL of 0.1 N iodine VS. Titrate with 0.1 N sodium thiosulfate VS, adding 1 mL of starch TS near the endpoint. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ). Each mL of sodium thiosulfate is equivalent to 16.42 mg of C2H5NaO3S2.
). Each mL of sodium thiosulfate is equivalent to 16.42 mg of C2H5NaO3S2.
Acceptance criteria:
96.0%–102.0% on the dried basis
IMPURITIES
Inorganic Impurities
• Limit of Chloride
Chloride standard solution:
8.24 µg/mL of sodium chloride in water
Sample solution:
200 mg/mL of Mesna in carbon dioxide-free water
Analysis:
To 1 mL of the Sample solution and 15 mL of water add 1 mL of 2 M nitric acid. Add the resulting solution to 1 mL of a silver nitrate solution (17 g in 1000 mL), and allow to stand for 5 min, protected from light. To 10 mL of the Chloride standard solution add 5 mL of water and 1 mL of 2 M nitric acid. To this solution add 1 mL of silver nitrate solution (17 g in 1000 mL) and allow to stand for 5 min, protected from light. When viewed against a dark background, the Sample solution is not more turbid than the Chloride standard solution.
Acceptance criteria:
NMT 250 ppm
• Limit of Sulfate
Sulfate standard solution:
1.81 mg/mL of potassium sulfate in alcohol. Immediately before use, dilute with alcohol to 100 times its volume, and mix.
Sample solution:
Add 5.0 mL of the Sample solution prepared as directed in the test for Limit of Chloride to a 30-mL volumetric flask, and dilute with water to volume.
Analysis:
Add 3 mL of a 250-g/L solution of barium chloride to 4.5 mL of Sulfate standard solution. Shake and allow to stand for 1 min. To 2.5 mL of this solution add 15 mL of the Sample solution and 0.5 mL of acetic acid. Use 15 mL of this mixture for comparison with 15 mL of the Sulfate standard solution, prepared in the same manner, but using the Sulfate standard solution instead of the Sample solution. After 5 min, any opalescence in the Sample solution is not more intense than that in the Sulfate standard solution.
Acceptance criteria:
NMT 300 ppm
• Heavy Metals, Method I  231
231 :
10 ppm
:
10 ppm
Organic Impurities
• Procedure
Mobile phase:
In a 1000-mL volumetric flask dissolve 2.94 g of potassium dihydrogen phosphate, 2.94 g of dipotassium hydrogen phosphate, and 2.6 g of tetrabutylammonium hydrogen sulfate in about 600 mL of water. Adjust to a pH of 2.3 with phosphoric acid, add 335 mL of methanol, and dilute with water to volume.
System suitability solution:
0.18 mg/mL and 0.004 mg/mL of USP Mesna RS and USP Mesna Related Compound A RS, respectively, in Mobile phase
Standard solution 1:
8 µg/mL and 120 µg/mL of USP Mesna Related Compound A RS and USP Mesna Related Compound B RS, respectively, in Mobile phase
Standard solution 2:
12 µg/mL of USP Mesna RS in Mobile phase
Sample solution:
4 mg/mL of Mesna in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 235 nm
Column:
4.6-mm × 25-cm; 10-µm packing L1
Flow rate:
1 mL/min
Run time:
Four times the elution time for mesna
Injection size:
20 µL
System suitability
Sample:
System suitability solution
[Note—The relative retention times for mesna and mesna related compound A are about 1.0 and 1.4, respectively. ]
Suitability requirements
Resolution:
NLT 3.0 between mesna and mesna related compound A
Analysis
Samples:
Standard solution 1, Standard solution 2, and Sample solution
[Note—Identify the peaks using the relative retention times provided in Impurity Table 1. ]
Calculate the percentage of mesna related compound A in the portion of Mesna taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of mesna related compound A from the Sample solution |
| rS | = | = peak response of mesna related compound A from Standard solution 1 |
| CS | = | = concentration of USP Mesna Related Compound A RS in Standard solution 1 (mg/mL) |
| CU | = | = nominal concentration of Mesna in the Sample solution (mg/mL) |
Calculate the percentage of mesna related compound B in the portion of Mesna taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of mesna related compound B from the Sample solution |
| rS | = | = peak response of mesna related compound B from Standard solution 1 |
| CS | = | = concentration of USP Mesna Related Compound B RS in Standard solution 1 (mg/mL) |
| CU | = | = nominal concentration of Mesna in the Sample solution (mg/mL) |
Calculate the percentage of any specified impurities (2-(carbamimidoylsulphanyl)ethanesulfonic acid;2-[[(guanidino)(imino)methyl]sulphanyl]ethanesulfonic acid; 2-(4,6-diamino-1,3,5-triazin-2-yl)sulphanylethanesulfonic acid) and any unspecified impurities in the portion of Mesna taken:
Result = (rU/rS) × (CS/CU) × F × 100
| rU | = | = peak response of any specified or unspecified individual impurity from the Sample solution |
| rS | = | = peak response of mesna from Standard solution 2 |
| CS | = | = concentration of USP Mesna RS in Standard solution 2 (mg/mL) |
| CU | = | = nominal concentration of Mesna in the Sample solution (mg/mL) |
| F | = | = relative response factors (see Impurity Table 1) |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| 2-(Carbamimidoylsulfanyl)ethanesulfonic acid | 0.6 | 100 | 0.3 |
| 2-[[(Guanidino) (imino)methyl] sulfanyl]ethanesulfonic acid |
0.6 | 100 | 0.3 |
| 2-(4,6-Diamino- 1,3,5-triazin-2-yl)sulfanylethanesulfonic acid |
0.8 | 100 | 0.3 |
| Mesna | 1.0 | — | — |
| Mesna related compound Aa | 1.4 | — | 0.2 |
| Mesna related compound Bb | 2.3 | — | 3.0 |
| Individual unspecified impurities | — | — | 0.1 |
| Total unspecified impurities | — | — | 0.3 |
|
a
2-(Acetysulfanyl)ethanesulfonic acid.
b
2,2-(Disulfanediyl)bis(ethanesulfonic acid).
|
|||
SPECIFIC TESTS
• Loss on Drying:
Dry 1 g in a vacuum at a pressure not exceeding 1 mm of mercury at 60 over phosphorus pentoxide for 2 h: it loses NMT 1.0% of its weight.
over phosphorus pentoxide for 2 h: it loses NMT 1.0% of its weight.
• pH  791
791 :
4.5–6.0
:
4.5–6.0
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in a tight container, and store at room temperature.
• USP Reference Standards  11
11
USP Mesna Related Compound A RS
2-(Acetysulfanyl)ethanesulfonic acid.
C4H8O4S2 184.23
2-(Acetysulfanyl)ethanesulfonic acid.
C4H8O4S2 184.23
USP Mesna Related Compound B RS
2,2-(Disulfanediyl)bis(ethanesulfonic acid).
C4H10O6S4 282.38
2,2-(Disulfanediyl)bis(ethanesulfonic acid).
C4H10O6S4 282.38
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3820
Pharmacopeial Forum: Volume No. 34(5) Page 1168