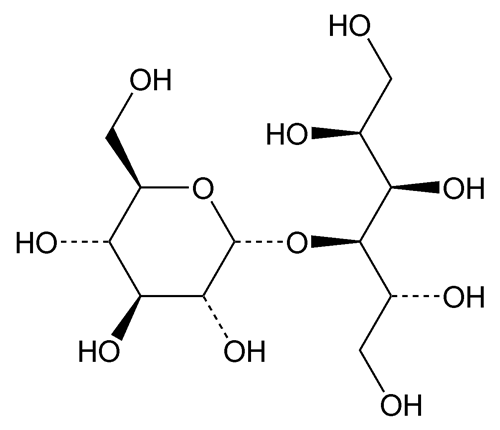
Maltitol
(mawl' ti tol).
DEFINITION
Maltitol contains NLT 92.0% and NMT 100.5% of d-maltitol, calculated on the anhydrous basis. The amounts of total sugars, other polyhydric alcohols, and any polyol anhydrides, if detected, are not included in the requirements or in the calculated amount under General Notices and Requirements, Other Impurities.
IDENTIFICATION
• A. Procedure
Sample solution:
1 g of Maltitol in 75 mL of water
Analysis:
Transfer 3 mL of the Sample solution to a 15-cm test tube, and add 3 mL of freshly prepared catechol solution (1 in 10). Add 6 mL of sulfuric acid, mix again, and gently heat the tube in a flame for 30 s.
Acceptance criteria:
A deep pink or wine-red color appears.
• B.
The retention time of the major peak from the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Mobile phase:
Water. [Note—Degas the Mobile phase before use. ]
System suitability solution:
4.8 mg/g of USP Maltitol RS and 4.8 mg/g of sorbitol
Standard solution:
10 mg/g of USP Maltitol RS and 1.6 mg/g of sorbitol
Sample solution:
Dissolve 0.20 g of Maltitol in water, and dilute with water to 20 g. Record the final solution weight, and mix thoroughly.
Chromatographic system
Mode:
LC
Detector:
Refractive index
Column:
7.8-mm × 10-cm; packing L34
Temperature
Column:
60 ± 2
Detector:
35
Flow rate:
0.5 mL/min
Injection size:
10 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for maltitol and sorbitol are 0.48 and 1.0, respectively. ]
Suitability requirements
Resolution:
NLT 2.0 between maltitol and sorbitol, System suitability solution
Relative standard deviation:
NMT 2.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of maltitol (C12H24O11) in the portion of Maltitol taken:
Result = (rU/rS) × (CS/CU) × [100/(100  W)] × 100
W)] × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Maltitol RS in the Standard solution (mg/g) |
| CU | = | = concentration of Maltitol in the Sample solution (mg/g) |
| W | = | = percentage obtained in the test for Water Determination |
Acceptance criteria:
92.0%–100.5% on the anhydrous basis
IMPURITIES
• Limit of Nickel
Sample solution:
Dissolve 20.0 g of Maltitol in diluted acetic acid, and dilute with diluted acetic acid to 150 mL.
Blank solution:
150 mL of diluted acetic acid
Standard solutions:
Prepare three solutions by adding 0.5, 1.0, and 1.5 mL of nickel standard solution TS to 20.0 g of Maltitol dissolved in diluted acetic acid, and dilute with the same solvent to 150 mL.
Instrumental conditions
Mode:
Atomic absorption spectrophotometry
Analytical wavelength:
Maxima at about 232.0 nm
Lamp:
Nickel hollow-cathode
Flame:
Air–acetylene
Analysis
Samples:
Standard solutions and Sample solution
To each sample, add 2.0 mL of a saturated ammonium pyrrolidinedithiocarbamate solution (containing 10 g/L of ammonium pyrrolidinedithiocarbamate) and 10.0 mL of methyl isobutyl ketone, and shake for 30 s. Protect from bright light. Allow the two layers to separate, and use the methyl isobutyl ketone layer. Set the instrument to zero using the organic layer from the Blank solution. Concomitantly determine the absorbances of the organic layer from the Samples at least three times each. Record the average of the steady readings for each of the Standard solutions and the Sample solution. Between each measurement, aspirate the organic layer from the Blank solution, and ascertain that the reading returns to zero. Plot the absorbances of the Standard solutions and the Sample solution versus the added quantity of nickel. Extrapolate the line joining the points on the graph until it meets the concentration axis. The distance between this point and the intersection of the axes represents the concentration of nickel in the Sample solution.
Acceptance criteria:
NMT 1 ppm
• Reducing Sugars
Sample:
3.3 g
Analysis:
Dissolve the Sample in 3 mL of water with the aid of gentle heat. Cool, and add 20.0 mL of cupric citrate TS and a few glass beads. Heat so that boiling begins after 4 min, and maintain boiling for 3 min. Cool rapidly, and add 40 mL of diluted acetic acid, 60 mL of water, and 20.0 mL of 0.05 N iodine VS. With continuous shaking, add 25 mL of hydrochloric acid in water solution (6:94). When the precipitate has dissolved, titrate the excess of iodine with 0.05 N sodium thiosulfate VS using 2 mL of starch TS, added towards the end of the titration, as an indicator.
Acceptance criteria:
NLT 12.8 mL of 0.05 N sodium thiosulfate VS is required, corresponding to NMT 0.3% of reducing sugars, as glucose.
[Note—The amount determined in this test is not included in the calculated amount under General Notices and Requirements, Other Impurities. ]
SPECIFIC TESTS
• Microbial Enumeration Tests  61
61 and Tests for Specified Microorganisms
and Tests for Specified Microorganisms  62
62 :
The total aerobic microbial count using the Plate Method does not exceed 1000 cfu/g, and the total combined molds and yeasts count does not exceed 100 cfu/g.
:
The total aerobic microbial count using the Plate Method does not exceed 1000 cfu/g, and the total combined molds and yeasts count does not exceed 100 cfu/g.
• Conductivity
Sample solution:
200 mg/mL
Analysis:
Using an appropriate conductivity meter, choose a conductivity cell that is appropriate for the properties and conductivity of the solution to be examined. Use a certified reference material, for example a solution of potassium chloride, that is appropriate for the measurement.1
The conductivity value of the certified reference material should be near the expected conductivity value of the solution to be examined. After calibrating the apparatus with a certified reference material solution, rinse the conductivity cell several times with water and at least twice with the aqueous solution to be examined. Measure the conductivity of the Sample solution at a temperature of 20 , while gently stirring with a magnetic stirrer.
, while gently stirring with a magnetic stirrer.
Acceptance criteria:
NMT 20 µS/cm
• Water Determination, Method I  921
921 :
NMT 1.0%
:
NMT 1.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers. No storage requirements are specified.
1
Commercially available conductivity calibration solutions for conductivity meter standardization, standardized by methods traceable to the National Institute of Standards and Technology (NIST), may be used. Solutions prepared according to instructions given in ASTM Standard D1125 may be used provided the conductivity of the resultant solution is the same as that of the solution prepared from the NIST-certified material.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 1852
Pharmacopeial Forum: Volume No. 31(4) Page 1143