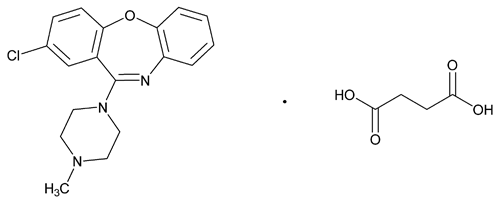
Loxapine Succinate
C18H18ClN3O·C4H6O4 445.90
Butanedioic acid, compd. with 2-chloro-11-(4-methyl-1-piperazinyl)dibenz[b,f][1,4]oxazepine (1:1);
2-Chloro-11-(4-methyl-1-piperazinyl)dibenz[b,f][1,4] oxazepine succinate (1:1)

 [27833-64-3].
[27833-64-3].
(lox' a peen sux' i nate).
C18H18ClN3O·C4H6O4 445.90
Butanedioic acid, compd. with 2-chloro-11-(4-methyl-1-piperazinyl)dibenz[b,f][1,4]oxazepine (1:1);
2-Chloro-11-(4-methyl-1-piperazinyl)dibenz[b,f][1,4] oxazepine succinate (1:1)
DEFINITION
Loxapine Succinate contains NLT 98.5% and NMT 101.0% of C18H18ClN3O·C4H6O4, calculated on the dried basis.
IDENTIFICATION
• B. Ultraviolet Absorption  197U
197U
Sample solution:
20 µg/mL in 0.01 N hydrochloric acid
ASSAY
• Procedure
Sample:
400 mg of Loxapine Succinate
Analysis:
Dissolve in 80 mL of glacial acetic acid, and titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction (see Titrimetry  541
541 ).
).
Calculate the percentage of C18H18ClN3O·C4H6O4 in the portion of Loxapine Succinate taken:
Result = [(V  B) × N × F × 100]/W
B) × N × F × 100]/W
| V | = | = sample titrant volume (mL) |
| B | = | = blank titrant volume (mL) |
| N | = | = normality of titrant (mEq/mL) |
| F | = | = equivalence factor, 22.29 mg/mEq |
| W | = | = sample weight (mg) |
Acceptance criteria:
98.5%–101.0% on the dried basis
IMPURITIES
Organic Impurities
• Procedure
Buffer:
3.9 g/L of ammonium acetate in water. Adjust with 20% acetic acid in water or 6 N ammonium hydroxide to a pH of 7.3.
Mobile phase:
Acetonitrile and Buffer (3:7)
Diluent:
Acetonitrile and Buffer (7:3)
Standard solution:
10 µg/mL of USP Loxapine Succinate RS and 1.5 µg/mL each of USP Amoxapine RS and USP Loxapine Related Compound A RS in Diluent. [Note—Amoxapine has been included in the Standard solution for peak identification purposes only. ]
Sample solution:
1 mg/mL of Loxapine Succinate in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 254 nm
Column:
4.6-mm × 5-cm; 2.7-µm packing L1
Column temperature:
35
Flow rate:
1.5 mL/min
Injection size:
10 µL
Run time:
2 times the retention time of loxapine succinate
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 2.0 between loxapine succinate and loxapine related compound A
Relative standard deviation:
NMT 5.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of any individual impurity in the portion of Loxapine Succinate taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of loxapine succinate from the Standard solution |
| CS | = | = concentration of USP Loxapine Succinate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Loxapine Succinate in the Sample solution (mg/mL) |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.5%
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Amoxapinea | 0.19 | 1.3 | 0.15 |
| Chlorodibenzoxazepinoneb | 0.45 | 0.70 | 0.15 |
| Loxapine succinate | 1.0 | — | — |
| Loxapine related compound Ac | 1.2 | 1.2 | 0.15 |
| Any unspecified individual impurity | — | 1.0 | 0.10 |
|
a
2-Chloro-11-(piperazin-1-yl)dibenzo[b,f][1,4]oxazepine.
b
2-Chlorodibenzo[b,f]-1,4-oxazepin-11-one.
c
3-Chloro-11-(4-methylpiperazin-1-yl)dibenzo[b,f][1,4]oxazepine.
|
|||
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 4 h: it loses NMT 0.5% of its weight.
for 4 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• USP Reference Standards  11
11
USP Loxapine Related Compound A RS
3-Chloro-11-(4-methylpiperazin-1-yl)dibenzo[b,f][1,4]oxazepine.
C18H18ClN3O 327.81
3-Chloro-11-(4-methylpiperazin-1-yl)dibenzo[b,f][1,4]oxazepine.
C18H18ClN3O 327.81
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3730
Pharmacopeial Forum: Volume No. 36(4) Page 924