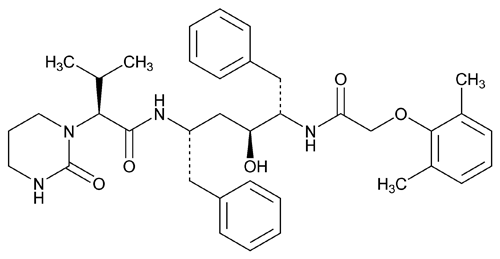
Lopinavir
C37H48N4O5 628.80
[1S-[1R*(R*),3R*,4R*]]-N-[4[[(2,6-Dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]-tetrahydro- -(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide;
-(1-methylethyl)-2-oxo-1(2H)-pyrimidineacetamide;
( S)-Tetrahydro-N-[(
S)-Tetrahydro-N-[( S)-
S)- -[(2S,3S)-2-hydroxy-4-phenyl-3-[2-(2,6-xylyloxy)acetamido]butyl]phenethyl]-
-[(2S,3S)-2-hydroxy-4-phenyl-3-[2-(2,6-xylyloxy)acetamido]butyl]phenethyl]- -isopropyl-2-oxo-1(2H)-pyrimidineacetamide
-isopropyl-2-oxo-1(2H)-pyrimidineacetamide


 [192725-17-0].
[192725-17-0].
(loe pin' a vir).
C37H48N4O5 628.80
[1S-[1R*(R*),3R*,4R*]]-N-[4[[(2,6-Dimethylphenoxy)acetyl]amino]-3-hydroxy-5-phenyl-1-(phenylmethyl)pentyl]-tetrahydro-
(
DEFINITION
Lopinavir contains NLT 98.0% and NMT 102.0% of C37H48N4O5 calculated on the anhydrous basis.
IDENTIFICATION
• A. Infrared Absorption  197S
197S
Sample solution:
Dissolve 50 mg in 1.0 mL of deuterated chloroform.
• B.
The retention time of the lopinavir peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Buffer:
2.7 g/L of monobasic potassium phosphate and 0.9 g/L of dibasic potassium phosphate in water. Adjust with phosphoric acid to a pH of 6.0. Pass the solution through a suitable filter of 0.45-µm pore size.
Diluent:
Acetonitrile and water (1:1)
Solution A:
Acetonitrile and Buffer (9:11)
Mobile phase:
Solution A
Standard solution:
0.025 mg/mL of USP Lopinavir RS in Diluent
Sample solution:
0.025 mg/mL in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 215 nm
Column:
4.6-mm × 25-cm; 4-µm packing L1
Column temperature:
50
Flow rate:
1 mL/min
Injection size:
20 µL
Run time:
60 min
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 8000 theoretical plates
Capacity factor:
NLT 15
Tailing factor:
0.8–1.5
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C37H48N4O5 in the portion of Lopinavir taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of lopinavir from the Sample solution |
| rS | = | = peak response of lopinavir from the Standard solution |
| CS | = | = concentration of USP Lopinavir RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Lopinavir in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Organic Impurities
• Procedure 1
[Note—For early-eluting impurities. ]
Buffer, Diluent, and Solution A:
Proceed as directed in the Assay.
Solution B:
Acetonitrile and Buffer (3:1)
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 60 | 100 | 0 |
| 61 | 0 | 100 |
| 81 | 0 | 100 |
| 82 | 100 | 0 |
| 100 | 100 | 0 |
System suitability solution:
0.5 mg/mL of USP Lopinavir System Suitability Mixture RS in Diluent
Standard solution:
0.005 mg/mL of USP Lopinavir RS in Diluent
Sample solution:
0.5 mg/mL in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 215 nm
Column:
4.6-mm × 25-cm; 4-µm packing L1
Column temperature:
50
Flow rate:
1 mL/min
Injection size:
20 µL
Run time:
100 min
[Note—Data collection is only for the first 60 min. The remaining gradient steps wash out the late eluting impurities and re-equilibrate the column. ]
System suitability
Samples:
System suitability solution and Standard solution[Note—The relative retention times are listed in Impurity Table 1. ]
Suitability requirements
Resolution:
NLT 1.2 between lopinavir N-formylphenoxyacetamide and lopinavir N-acetylphenoxyacetamide, System suitability solution
Capacity factor:
NLT 15, Standard solution
Column efficiency:
NLT 8000, Standard solution
Tailing factor:
0.8–1.5, Standard solution
Relative standard deviation:
NMT 3.0%, Standard solution
Analysis
Samples:
Diluent, System suitability solution, Standard solution, and Sample solution
Calculate the percentage of each lopinavir related impurity and unidentified impurity in the portion of Lopinavir taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of lopinavir from the Standard solution |
| CS | = | = concentration of USP Lopinavir RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Lopinavir in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Impurity Table 1) |
Impurity Table 1
| Name | Relative Retention Timeo |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|---|
| Lopinavir free aminea | 0.03 | 0.61 | 0.1 | |
| Lopinavir N-formylaminoalcoholb | 0.07 | 0.80 | 0.2 | |
| Lopinavir divalinatec | 0.10 | 0.65 | 0.1 | |
| Sulfolopinavird | 0.13 | 0.76 | 0.1 | |
| Lopinavir phenoxyacetamidee | 0.25 | 0.96 | 0.1 | |
| Lopinavir N-formylphenoxyacetamidef | 0.59 | 1.3 | 0.1 | |
| Lopinavir N-acetylphenoxyacetamideg | 0.62 | 1.2 | 0.1 | |
| Lopinavir oxazineh | 0.90 | 1.1 | 0.1 | |
| Lopinavir | 1.00 | — | — | |
| Isolopinaviri | 1.10 | 0.99 | 0.2 | |
| Lopinavir 2,4-phenoxy isomerj | 1.13 | 0.97 | 0.1 | |
| Lopinavir d-leucine diastereomerk | 1.25 | 1.1 | 0.1 | |
| Z-Diacylethenediaminel | 1.28 | 1.4 | 0.1 | |
| Lopinavir (2R,4R) diastereomerm | 1.32 | 1.0 | 0.1 | |
| Lopinavir (4R) epimern | 1.38 | 0.97 | 0.1 | |
| Any other individual impurity | — | 1.0 | 0.1 | |
|
a
(S)-N-[(2S,4S,5S)-5-Amino-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
b
(S)-N-[(2S,4S,5S)-5-Formamido-4-hydroxy-1,6-diphenylhexan-2-yl]-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
c
(2S,2'S)-N,N'-[(2S,3S,5S)-3-Hydroxy-1,6-diphenylhexane-2,5-diyl]bis{3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide}.
d
(2S,3S,5S)-2-[2-(2,6-Dimethylphenoxy)acetamido]-5-{(S)-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamido}-1,6-diphenylhexan-3-yl hydrogen sulfate.
e
N-[(2S,3S,5S)-5-Amino-3-hydroxy-1,6-diphenylhexan-2-yl]-2-(2,6-dimethylphenoxy)acetamide.
f
2-(2,6-Dimethylphenoxy)-N-[(2S,3S,5S)-5-formamido-3-hydroxy-1,6-diphenylhexan-2-yl]acetamide.
g
N-[(2S,3S,5S)-5-Acetamido-3-hydroxy-1,6-diphenylhexan-2-yl]-2-(2,6-dimethylphenoxy)acetamide.
h
N-{(S)-1-[(4S,6S)-4-Benzyl-2-oxo-1,3-oxazinan-6-yl]-2-phenylethyl}-2-(2,6-dimethylphenoxy)acetamide.
i
(S)-N-{(2S,3S,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-3-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
j
(S)-N-{(2S,4S,5S)-5-[2-(2,4-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
k
(R)-N-{(2S,4S,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
l
(Z)-N,N'-(Ethene-1,2-diyl)bis[2-(2,6-dimethylphenoxy)acetamide].
m
(S)-N-{(2R,4R,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
n
(S)-N-{(2S,4R,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
o
(See Chromatography
|
||||
• Procedure 2
[Note—For late-eluting impurities. ]
Buffer, Diluent, and Solution A:
Proceed as directed in the Assay.
Solution B:
Acetonitrile and Buffer (3:1)
Mobile phase:
Solution A and Solution B (3:7)
System suitability solution:
0.5 mg/mL of USP Lopinavir System Suitability Mixture RS in Diluent
Standard solution:
0.005 mg/mL of USP Lopinavir RS in Diluent
Sample solution:
0.5 mg/mL in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 215 nm
Column:
4.6-mm × 25-cm; 4-µm packing L1
Column temperature:
50
Flow rate:
1 mL/min
Injection size:
20 µL
Run time:
50 min
System suitability
Suitability requirements
Capacity factor:
NLT 1.5
Column efficiency:
NLT 3000
Tailing factor:
0.8–1.5
Relative standard deviation:
NMT 3.0%
Analysis
Samples:
Diluent, System suitability solution, Standard solution, and Sample solution
Calculate the percentage of each lopinavir related impurity and unidentified impurity in the portion of Lopinavir taken:
Result = (rU/rS) × (CS/CU) × (1/F) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of lopinavir from the Standard solution |
| CS | = | = concentration of USP Lopinavir RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Lopinavir in the Sample solution (mg/mL) |
| F | = | = relative response factor (see Impurity Table 2) |
Impurity Table 2
| Name | Relative Retention Timeg |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|---|
| Lopinavir | 1.00 | — | — | |
| Lopinavir O-acyla | 1.49 | 0.77 | 0.1 | |
| Lopinavir (2R) epimerb | 1.91 | 1.1 | 0.1 | |
| Lopinavir diamidec | 4.39 | 1.4 | 0.1 | |
| Lopinavir N-acyld | 6.01 | 1.3 | 0.1 | |
| Lopinavir O-phenoxyactyle | 7.14 | 1.1 | 0.1 | |
| Lopinavir aminoalcohol ureaf | 8.46 | 1.3 | 0.1 | |
| Any other individual impurity | — | 1.0 | 0.1 | |
|
a
(S)-{(2S,3S,5S)-2-[2-(2,6-Dimethylphenoxy)acetamido]-5-[(S)-3-methyl-2-(2-oxotetrahydropyrimidin-1(2H)-yl)butanamido]-1,6-diphenylhexan-3-yl} 3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanoate.
b
(S)-N-{(2R,4S,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamide.
c
N,N'-[(2S,3S,5S)-3-Hydroxy-1,6-diphenylhexane-2,5-diyl]bis[2-(2,6-dimethylphenoxy)acetamide].
d
(S)-N-{(2S,4S,5S)-5-[2-(2,6-Dimethylphenoxy)acetamido]-4-hydroxy-1,6-diphenylhexan-2-yl}-2-{3-[2-(2,6-dimethylphenoxy)acetyl]-2-oxotetrahydropyrimidin-1(2H)-yl}-3-methylbutanamide.
e
(2S,3S,5S)-2-[2-(2,6-Dimethylphenoxy)acetamido]-5-{(S)-3-methyl-2-[2-oxotetrahydropyrimidin-1(2H)-yl]butanamido}-1,6-diphenylhexan-3-yl 2-(2,6-dimethylphenoxy)acetate.
f
N,N'-(2S,2'S,3S,3'S,5S,5'S)-5,5'-Carbonylbis(azanediyl)bis(3-hydroxy-1,6-diphenylhexane-5,2-diyl)bis[2-(2,6-dimethylphenoxy)acetamide].
g
(See Chromatography
|
||||
Acceptance criteria
Total impurities:
NMT 0.7%
[Note—Total impurities from Procedure 1 and Procedure 2. ]
SPECIFIC TESTS
• Water Determination, Method I  921
921 :
NMT 4.4%
:
NMT 4.4%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers. Store at room temperature.
• USP Reference Standards  11
11
USP Lopinavir System Suitability Mixture RS
Lopinavir N-formylphenoxyacetamide is (2-(2,6-dimethylphenoxy)-N-[(2S,3S,5S)-5-formamido-3-hydroxy-1,6-diphenylhexan-2-yl]acetamide.
C29H34N2O4 474.59
Lopinavir N-acetylphenoxyacetamide is (N-[(2S,3S,5S)-5-acetamido-3-hydroxy-1,6-diphenylhexan-2-yl]-2-(2,6-dimethylphenoxy)acetamide.
C30H36N2O4 488.62
Lopinavir System Suitability Mixture contains lopinavir N-formylphenoxyacetamide, lopinavir N-acetylphenoxyacetamide, and several other minor components.
Lopinavir N-formylphenoxyacetamide is (2-(2,6-dimethylphenoxy)-N-[(2S,3S,5S)-5-formamido-3-hydroxy-1,6-diphenylhexan-2-yl]acetamide.
C29H34N2O4 474.59
Lopinavir N-acetylphenoxyacetamide is (N-[(2S,3S,5S)-5-acetamido-3-hydroxy-1,6-diphenylhexan-2-yl]-2-(2,6-dimethylphenoxy)acetamide.
C30H36N2O4 488.62
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientific Liaison 1-301-816-8394 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3706
Pharmacopeial Forum: Volume No. 36(3) Page 671