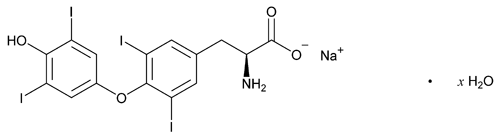
Levothyroxine Sodium
C15H10I4NNaO4·xH2O (anhydrous) 798.85
l-Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-, monosodium salt, hydrate;
Monosodium l-thyroxine hydrate

 [25416-65-3].
[25416-65-3].
Anhydrous

 [55-03-8].
[55-03-8].
(lee'' voe thye rox' een soe' dee um).
C15H10I4NNaO4·xH2O (anhydrous) 798.85
l-Tyrosine, O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-, monosodium salt, hydrate;
Monosodium l-thyroxine hydrate
Anhydrous
DEFINITION
Levothyroxine Sodium is the sodium salt of l-3,3¢,5,5¢-tetraiodothyronine. It contains NLT 97.0% and NMT 103.0% of C15H10I4NNaO4, calculated on the anhydrous basis.
IDENTIFICATION
• A.
Sample:
50 mg
Analysis:
Ignite the Sample in a platinum dish over a flame.
Acceptance criteria:
It decomposes and liberates iodine vapors. [Note—Cool the residue, and reserve it for use in Identification test D. ]
• B.
Acid sodium chloride solution:
Alcohol, 1 N sodium hydroxide, hydrochloric acid, and water (25:10:10:30)
Sample:
0.5 mg
Analysis:
Add 7.5 mL of Acid sodium chloride solution and 1 mL of 10 mg/mL sodium nitrite solution to the Sample. Allow to stand in the dark for 20 min, and add 1.25 mL of ammonium hydroxide.
Acceptance criteria:
A pink color is produced.
• C.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• D. Identification Tests—General, Sodium  191
191 :
The solution meets the requirements of the flame test.
:
The solution meets the requirements of the flame test.
Sample solution:
To the residue retained from Identification test A, add a 1 N potassium hydroxide solution dropwise until the residue is dissolved.
ASSAY
• Procedure
Mobile phase:
Acetonitrile and water (4:6) that contains 0.5 mL of phosphoric acid in each 1000 mL
Solution A:
400 mg of sodium hydroxide in 500 mL of water. Cool and add 500 mL of methanol.
Levothyroxine stock solution:
0.4 mg/mL of USP Levothyroxine RS in Solution A
Liothyronine stock solution:
0.4 mg/mL of liothyronine from USP Liothyronine RS in Solution A. Make a 1:100 dilution of this solution using Mobile phase.
Standard solution:
10 µg/mL of levothyroxine from Levothyroxine stock solution and 0.2 µg/mL of liothyronine from Liothyronine stock solution, in Mobile phase
Sample solution:
Prepare a solution of Levothyroxine Sodium in Mobile phase having a known concentration of 10 µg/mL. [Note—A small amount of 0.01 M methanolic sodium hydroxide can be used to facilitate the dissolution of the sample. ]
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm × 25-cm; packing L10
Flow rate:
1.5 mL/min
Injection size:
100 µL
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 5.0 between liothyronine and levothyroxine
Relative standard deviation:
NMT 2.0% for levothyroxine
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of levothyroxine sodium (C15H10I4NNaO4) in the portion of Levothyroxine Sodium taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response of levothyroxine from the Sample solution |
| rS | = | = peak response of levothyroxine from the Standard solution |
| CS | = | = concentration of USP Levothyroxine RS in the Standard solution (µg/mL) |
| CU | = | = concentration of Levothyroxine Sodium in the Sample solution (µg/mL) |
| Mr1 | = | = molecular weight of levothyroxine sodium, 798.85 |
| Mr2 | = | = molecular weight of levothyroxine, 776.87 |
Acceptance criteria:
97.0%–103.0% on the anhydrous basis
IMPURITIES
[Note—On the basis of the synthetic route, perform either Organic Impurities, Procedure 1 or Organic Impurities, Procedure 2. Procedure 2 is recommended when related compounds listed in Table 3 may be present. ]
• Organic Impurities, Procedure 1
Diluent:
Acetonitrile and water (1:1)
Solution A:
Dilute 5 mL of phosphoric acid with Diluent to 100.0 mL.
Mobile phase:
Dissolve 1.0 g of sodium 1-heptanesulfonate in 200 mL of water. Add 200 mL of acetonitrile, 400 mL of methanol, and 1.0 mL of phosphoric acid. Dilute with water to 1 L.
Standard stock solution 1:
Transfer 25 mg of USP Levothyroxine RS to a 100-mL volumetric flask. Add 50 mL of Diluent and 1 drop of 10 N sodium hydroxide, and sonicate until dissolved. Add 7 mL of Solution A, and dilute with Diluent to volume.
Standard stock solution 2:
Transfer 25 mg of USP Liothyronine RS to a 100-mL volumetric flask. Add 50 mL of Diluent and 1 drop of 10 N sodium hydroxide, and sonicate until dissolved. Add 7 mL of Solution A, and dilute with Diluent to volume.
System suitability solution:
Transfer 5.0 mL of Standard stock solution 1 and 5.0 mL of Standard stock solution 2 to a 100-mL volumetric flask. Add 7 mL of Solution A, and dilute with Diluent to volume.
Standard solution:
Pipet 4.0 mL of the System suitability solution into a 100-mL volumetric flask. Add 7 mL of Solution A, and dilute with Diluent to volume.
Blank solution:
Transfer 7 mL of Solution A to a 100-mL volumetric flask, and dilute with Diluent to volume.
Sample solution:
Transfer 25 mg of Levothyroxine Sodium to a 100-mL volumetric flask. Add 50 mL of Diluent, and sonicate until dissolved. Add 7 mL of Solution A, and dilute with Diluent to volume.
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm × 15-cm; 5-µm packing L7
Column temperature:
35
Flow rate:
1.5 mL/min
Injection size:
15 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 5.0 between levothyroxine and liothyronine, System suitability solution
Relative standard deviation:
NMT 2.0% for the levothyroxine peak, Standard solution
Analysis
Samples:
Standard solution, Blank solution, and Sample solution
[Note—Record the chromatograms for at least six times the retention time of the levothyroxine peak. Verify that no peaks elute in the Blank solution at the expected retention times for levothyroxine and related compounds. ]
Calculate the area percentage of each related compound in the portion of Levothyroxine Sodium taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of levothyroxine from the Standard solution |
| CS | = | = concentration of levothyroxine in the Standard solution (mg/mL) |
| CU | = | = concentration of Levothyroxine Sodium in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of levothyroxine sodium, 798.85 |
| Mr2 | = | = molecular weight of levothyroxine, 776.87 |
[Note—The relative response factor for the impurities listed in Table 1 is 1.00. Any unspecified impurity peaks should be assigned a relative response factor of 1.00. ]
Disregard peaks corresponding to those of the Blank solution, and disregard peaks corresponding to less than 0.03%.
Acceptance criteria:
See Table 1.
Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Liothyronine | 0.65–0.70 | 1.0 |
| 0.71–0.76 | 0.15 | |
| Levothyroxine | 1.0 | — |
| T4-Hydroxyacetic acidb | 1.13–1.28 | 0.15 |
| N-Formyl-T4c and T4-acetamided | 1.47–1.53 | 0.15 |
| N-Acetyl-T4e | 1.50–1.86 | 0.20 |
| T4-Acetic acidf | 2.42–2.51 | 0.30 |
| T4-Aldehydeg | 3.17–3.45 | 0.15 |
| T4-Benzoic acidh | 3.46–3.70 | 0.15 |
| Individual unspecified impurity | — | 0.10 |
| Total impurities | — | 2.0 |
|
a
O-(4-Hydroxy-3,5-diiodophenyl)-3,5-diiodo-
b
2-Hydroxy-2-(4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl)acetic acid.
c
N-Formyl-O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-l-tyrosine.
d
2-(4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl) acetamide.
e
N-Acetyl-O-(4-hydroxy-3,5-diiodophenyl)-3,5-diiodo-l-tyrosine.
f
2-(4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl)acetic acid.
g
4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodobenzaldehyde.
h
4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodobenzoic acid.
|
||
• Organic Impurities, Procedure 2
Solution A:
Dissolve 9.7 g of sulfamic acid in 2000 mL of water. Add 1.5 g of sodium hydroxide, mix to dissolve, and adjust with 2 N sodium hydroxide to a pH of 2.0.
Solution B:
Acetonitrile
Diluent 1:
Methanol and Solution A (90:10)
Diluent 2:
Acetonitrile and Solution A (30:70); mix with Diluent 1 (1:1).
Mobile phase:
See Table 2.
Table 2
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 70 | 30 |
| 10 | 70 | 30 |
| 40 | 20 | 80 |
| 50 | 20 | 80 |
| 53 | 70 | 30 |
| 75 | 70 | 30 |
Blank solution:
Use Diluent 2.
Standard stock solution:
0.1 mg/mL each of USP Levothyroxine RS and USP Liothyronine RS in Diluent 1
Standard solution:
0.002 mg/mL of USP Levothyroxine RS and USP Liothyronine RS, prepared using the Standard stock solution in Diluent 2
Sensitivity solution:
0.0002 mg/mL of USP Levothyroxine RS and USP Liothyronine RS, prepared using the Standard solution in Diluent 2
Identification solution:
Dissolve 5.0 mg of USP Levothyroxine for Peak Identification RS in 4.5 mL of methanol. Add 0.5 mL of Solution A. Further dilute a portion of this solution with Diluent 2 to obtain a solution containing about 0.2 mg/mL.
Sample solution:
Dissolve an amount of Levothyroxine Sodium in Diluent 1 to obtain a solution having a known concentration of about 1.0 mg/mL. Further dilute a portion of this solution with Diluent 2 to obtain a solution having a known concentration of about 0.2 mg/mL.
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.0-mm × 15-cm; 3-µm packing L1
Flow rate:
1.0 mL/min
Injection size:
25 µL
System suitability
Samples:
Standard solution and Sensitivity solution
Suitability requirements
Resolution:
NLT 5 between levothyroxine and liothyronine, Standard solution
Signal-to-noise ratio:
NLT 5 for each peak from the Sensitivity solution, calculated as follows:
Result = (2H)/h
| H | = | = measured height of the peak |
| h | = | = amplitude of the average measured baseline noise |
Analysis
Samples:
Blank solution, Standard solution, Identification solution, and Sample solution
[Note—Identify the components on the basis of their relative retention times as listed in Table 3. ]
Calculate the percentage of liothyronine sodium in the portion of Levothyroxine Sodium taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response of liothyronine from the Sample solution |
| rS | = | = peak response of liothyronine from the Standard solution |
| CS | = | = concentration of liothyronine in the Standard solution (mg/mL) |
| CU | = | = concentration of Levothyroxine Sodium in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of liothyronine sodium, 672.96 |
| Mr2 | = | = molecular weight of liothyronine, 650.98 |
Calculate the percentage of any other impurity in the portion of Levothyroxine Sodium taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response of any impurity from the Sample solution |
| rS | = | = peak response of levothyroxine from the Standard solution |
| CS | = | = concentration of levothyroxine in the Standard solution (mg/mL) |
| CU | = | = concentration of Levothyroxine Sodium in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of levothyroxine sodium, 798.85 |
| Mr2 | = | = molecular weight of levothyroxine, 776.87 |
[Note—The relative response factor for the impurities listed in Table 3 is 1.00. Any unspecified impurity peaks should be assigned a relative response factor of 1.00. ]
Disregard peaks corresponding to those of the Blank solution, and disregard peaks corresponding to less than 0.03%.
Acceptance criteria:
See Table 3.
Table 3
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Liothyronine | 0.65 | 1.0 |
| Monochlorotriiodothyroninea | 0.94 | 0.15 |
| Levothyroxine N-methylamideb | 0.97 | 0.15 |
| Levothyroxine | 1.0 | — |
| Triiodothyroacetic acid, or T3-acetic acidc | 1.57 | 0.15 |
| O-(4-Hydroxy-3,5-diiodophenyl)thyroxine, or T6d | 1.61 | 0.50 |
| O-Methyl-tetraiodothyroethylamine, or T4-amine O-methyle | 1.76 | 0.30 |
| T4-Acetic acidf | 1.79 | 0.30 |
| Individual unspecified impurity | — | 0.10 |
| Total impurities | — | 2.0 |
|
a
(S)-2-Amino-3-[3-chloro-4-(4-hydroxy-3,5-diiodophenoxy)-5-iodophenyl]
propanoic acid.
b
(S)-2-Amino-3-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl]-N-methylpropanamide.
c
[4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenyl]acetic acid.
d
(S)-2-Amino-3-[4-[4-(4-hydroxy-3,5-diiodophenoxy)-3,5-diiodophenoxy]-3,5-diiodophenyl]propanoic acid.
e
2-[4-(3,5-Diiodo-4-methoxyphenoxy)-3,5-diiodophenyl]ethanamine.
f
2-(4-(4-Hydroxy-3,5-diiodophenoxy)-3,5-diiodophenyl)acetic acid.
|
||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S :
:
 5
5 to
to  6
6
Sample solution:
Equivalent to 30 mg/mL of anhydrous Levothyroxine Sodium in alcohol and 1 N sodium hydroxide (2:1)
• Water Determination, Method I  921
921 :
NMT 11.0%
:
NMT 11.0%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, protected from light. Store as stated in the labeling instructions.
• Labeling:
If a test for Organic Impurities other than Procedure 1 is used, the labeling states the test with which the article complies.
• USP Reference Standards  11
11
USP Levothyroxine for Peak Identification RS
Levothyroxine sodium spiked with liothyronine, triiodothyroacetic acid, tetraiodothyroacetic acid.
Levothyroxine sodium spiked with liothyronine, triiodothyroacetic acid, tetraiodothyroacetic acid.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3676
Pharmacopeial Forum: Volume No. 35(3) Page 555