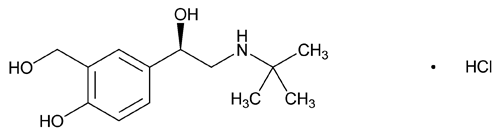
Levalbuterol Hydrochloride
C13H21NO3·HCl 275.77
(R)- 1-[(tert-Butylamino)methyl]-4-hydroxy-m-xylene-
1-[(tert-Butylamino)methyl]-4-hydroxy-m-xylene- ,
, ¢-diol hydrochloride
¢-diol hydrochloride


 [50293-90-8].
[50293-90-8].
(lev'' al bue' ter ol hye'' droe klor' ide).
C13H21NO3·HCl 275.77
(R)-
DEFINITION
Levalbuterol Hydrochloride contains NLT 98.0% and NMT 102.0% of C13H21NO3·HCl, calculated on the anhydrous basis.
IDENTIFICATION
ASSAY
• Procedure
Solution A:
1 in 1000 solution of phosphoric acid in water
Solution B:
Acetonitrile, methanol, phosphoric acid, and water (350:350:1:300)
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 91.5 | 8.5 |
| 15 | 91.5 | 8.5 |
| 15.01 | 0 | 100 |
| 20 | 0 | 100 |
| 20.01 | 91.5 | 8.5 |
| 30 | 91.5 | 8.5 |
Diluent:
Solution A
Standard solution:
100 µg/mL of USP Levalbuterol Hydrochloride RS in Diluent
Sample solution:
100 µg/mL of Levalbuterol Hydrochloride in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
35
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
Greater than 5500 theoretical plates
Tailing factor:
Less than 2.3
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C13H21NO3·HCl in the portion of Levalbuterol Hydrochloride taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of levalbuterol hydrochloride from the Sample solution |
| rS | = | = peak response of levalbuterol hydrochloride from the Standard solution |
| CS | = | = concentration of USP Levalbuterol Hydrochloride RS in the Standard solution (µg/mL) |
| CU | = | = concentration of the Sample solution (µg/mL) |
Acceptance criteria:
98.0%–102.0% on the anhydrous basis
IMPURITIES
Organic Impurities
• Procedure 1
Solution A, Solution B, Diluent, and Sample solution:
Proceed as directed in the Assay.
Standard solution:
[Note—Prepare a solution containing the following in Diluent. ]
USP Levalbuterol Hydrochloride RS, 100 µg/mL
USP Levalbuterol Related Compound A RS, 0.05 µg/mL
USP Levalbuterol Related Compound B RS, 0.05 µg/mL
USP Levalbuterol Related Compound C RS, 0.05 µg/mL
USP Levalbuterol Related Compound D RS, 0.05 µg/mL
USP Levalbuterol Related Compound E RS, 0.05 µg/mL
USP Levalbuterol Related Compound F RS, 0.05 µg/mL
USP Levalbuterol Related Compound H RS, 0.05 µg/mL
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 100 | 0 |
| 30 | 70 | 30 |
| 50 | 28 | 72 |
| 50.01 | 0 | 100 |
| 55 | 0 | 100 |
| 55.01 | 100 | 0 |
| 70 | 100 | 0 |
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
45
Flow rate:
1 mL/min
Injection size:
50 µL
System suitability
Sample:
Standard solution
Suitability requirements
Resolution:
NLT 4.9 between levalbuterol and levalbuterol related compound A; NLT 1.5 between levalbuterol related compound B and levalbuterol related compound C
Column efficiency:
NLT 4000 for levalbuterol
Tailing factor:
NMT 4.0 for levalbuterol
Relative standard deviation:
Less than 20% from any of the six related compound peaks
Analysis
Samples:
Standard solution and Sample solution
[Note—Integrate all peaks with an area greater than 0.05% of the area corresponding to the levalbuterol peak. ]
Calculate the percentage of each impurity in the portion of Levalbuterol Hydrochloride taken:
Result = (rU/rT) × (1/F) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rT | = | = sum of the responses of all the peaks |
| F | = | = relative response factor for each impurity (see Impurity Table 1) |
Acceptance criteria:
See Impurity Table 1.
Impurity Table 1
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|---|---|---|---|
| Levalbuterol related compound A | 1.2 | 1.0 | 0.1 |
| Levalbuterol related compound H | 1.3 | 1.0 | 0.15 |
| Levalbuterol related compound B | 1.5 | 1.0 | 0.10 |
| Levalbuterol related compound C | 1.6 | 1.0 | 0.15 |
| Levalbuterol related compound D | 1.7 | 3.0 | 0.05 |
| Levalbuterol related compound E | 2.1 | 1.0 | 0.1 |
| Levalbuterol related compound F | 3.5 | 1.2 | 0.10 |
| Any unknown impurity | — | — | 0.10 |
| Total unknown impurities | — | — | 0.1 |
| Total impurities | — | — | 0.5 |
• Procedure 2: Enantiomeric Purity and Chiral Identity
Mobile phase:
Acetonitrile, methanol, acetic acid, and triethylamine (500:500:3:1)
Diluent:
Mobile phase
System suitability solution A:
0.10 mg/mL of USP Levalbuterol Hydrochloride RS and 0.40 µg/mL of USP Albuterol RS in Diluent
System suitability solution B:
1.5 mg/mL of USP Albuterol RS in Diluent
Sample solution:
0.8 mg/mL of Levalbuterol Hydrochloride in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 225 nm
Column:
4.6-mm × 25-cm; 5-µm packing L63
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Sample:
System suitability solution A
Suitability requirements
Resolution:
NLT 2.0 between levalbuterol and (S)-albuterol
Column efficiency:
NLT 4000, calculated from either peak
Tailing factor:
NMT 2.2 for levalbuterol and (S)-albuterol
Relative standard deviation:
NMT 20% for (S)-albuterol, injected three times
Analysis
Samples:
System suitability solution B and Sample solution
Calculate the percentage of (S)-albuterol in the portion of Levalbuterol Hydrochloride taken:
Result = (rU/rT) × 100
| rU | = | = peak response of (S)-albuterol |
| rT | = | = sum of the peak responses for both levalbuterol and (S)-albuterol |
Acceptance criteria:
NMT 0.2% of (S)-albuterol
SPECIFIC TESTS
• Microbial Enumeration Tests  61
61 and Tests for Specified Microorganisms
and Tests for Specified Microorganisms  62
62 :
The total aerobic bacterial count is less than 10 cfu/g. The total combined molds and yeasts count is less than 10 cfu/g. It meets the requirements of the tests for absence of Salmonella species, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa.
:
The total aerobic bacterial count is less than 10 cfu/g. The total combined molds and yeasts count is less than 10 cfu/g. It meets the requirements of the tests for absence of Salmonella species, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa.
• pH  791
791 :
4.5–5.5, in a 10-mg/mL solution
:
4.5–5.5, in a 10-mg/mL solution
• Water Determination, Method Ic  921
921 :
NMT 0.3%
:
NMT 0.3%
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers, and store at controlled room temperature.
• USP Reference Standards  11
11
USP Levalbuterol Hydrochloride RS 
(R)- 1-[(tert-Butylamino)methyl]-4-hydroxy-m-xylene-
1-[(tert-Butylamino)methyl]-4-hydroxy-m-xylene- ,
, ¢-diol hydrochloride.
¢-diol hydrochloride.

(R)-
USP Levalbuterol Related Compound B RS 
 [{(1,1-Dimethylethyl)amino}methyl]-4-hydroxy-3-methyl-benzenemethanol.
[{(1,1-Dimethylethyl)amino}methyl]-4-hydroxy-3-methyl-benzenemethanol.

USP Levalbuterol Related Compound C RS 
 [{(1,1-Dimethylethyl)amino}methyl]-4-hydroxy-3-(methoxymethyl)-benzenemethanol.
[{(1,1-Dimethylethyl)amino}methyl]-4-hydroxy-3-(methoxymethyl)-benzenemethanol.

USP Levalbuterol Related Compound D RS 
5-[2-{(1,1-Dimethylethyl)amino]-1-hydroxyethyl]-2-hydroxy-benzaldehyde.

5-[2-{(1,1-Dimethylethyl)amino]-1-hydroxyethyl]-2-hydroxy-benzaldehyde.
USP Levalbuterol Related Compound E RS 
 [{(1,1-Dimethylethyl)amino}methyl]-3-(ethoxymethyl)-4-hydroxy-benzenemethanol.
[{(1,1-Dimethylethyl)amino}methyl]-3-(ethoxymethyl)-4-hydroxy-benzenemethanol.

USP Levalbuterol Related Compound F RS 
 [{(1,1-Dimethylethyl)amino}methyl]-4-(phenylmethoxy)-1,3-benzenedimethanol.
[{(1,1-Dimethylethyl)amino}methyl]-4-(phenylmethoxy)-1,3-benzenedimethanol.

USP Levalbuterol Related Compound H RS
4-[2-(tert-Butylamino)-1-methoxyethyl]-2-(hydroxymethyl)phenol.
C14H23NO3 253.34
4-[2-(tert-Butylamino)-1-methoxyethyl]-2-(hydroxymethyl)phenol.
C14H23NO3 253.34
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison 1-301-816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3654
Pharmacopeial Forum: Volume No. 36(3) Page 670