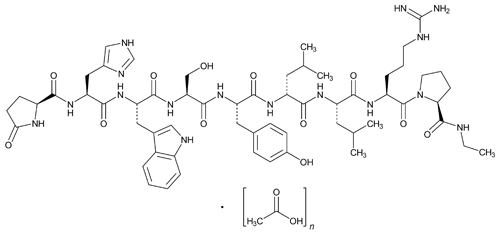
Leuprolide Acetate
C59H84N16O12·(C2H4O2)n, n = 1 or 2 1209.41 (as free base)
Luteinizing hormone-releasing factor, 6-d-leucine-9-(N-ethyl-l-prolinamide)-10-deglycinamide acetate (salt);
5-Oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-leucyl-l-leucyl-l-arginyl-N-ethyl-l-prolinamide acetate (salt)

 [74381-53-6].
[74381-53-6].
(loo' proe lide as' e tate).
C59H84N16O12·(C2H4O2)n, n = 1 or 2 1209.41 (as free base)
Luteinizing hormone-releasing factor, 6-d-leucine-9-(N-ethyl-l-prolinamide)-10-deglycinamide acetate (salt);
5-Oxo-l-prolyl-l-histidyl-l-tryptophyl-l-seryl-l-tyrosyl-d-leucyl-l-leucyl-l-arginyl-N-ethyl-l-prolinamide acetate (salt)
DEFINITION
Leuprolide Acetate is a synthetic nonapeptide agonist analog of luteinizing hormone-releasing factor. It contains NLT 97.0% and NMT 103.0% of leuprolide (C59H84N16O12), calculated on the anhydrous, acetic acid-free basis.
[Note—Due to the hygroscopic nature of this material, analyses are performed immediately after opening the container in a glove box under dry nitrogen purge. ]
[Caution—Leuprolide Acetate is a potent hormonal manipulator. Avoid skin contact and inhalation of dusts and mists.
]
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Solution A:
15.2 mg/mL of triethylamine in water. Adjust with phosphoric acid to a pH of 3.0.
Solution B:
Acetonitrile and n-propyl alcohol (3:2)
Mobile phase:
Solution A and Solution B (17:3)
Standard stock solution:
1 mg/mL of USP Leuprolide Acetate RS in Mobile phase
Standard solution:
50 µg/mL. Dilute 5.0 mL of the Standard stock solution with Mobile phase to 100.0 mL.
Degradation standard solution:
Dilute 5.0 mL of the Standard stock solution with water to 50.0 mL. Transfer 5 mL of the solution to a scintillation vial. Add 100 µL of 1 N sodium hydroxide solution, cap tightly, and shake vigorously. Place in an oven at 100 for 60 min. Remove, allow to cool, add 50 µL of 1 M phosphoric acid, recap, and shake vigorously to mix.
for 60 min. Remove, allow to cool, add 50 µL of 1 M phosphoric acid, recap, and shake vigorously to mix.
Sample solution:
50 µg/mL of Leuprolide Acetate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 10-cm; 3-µm packing L1
Flow rate:
1–1.5 mL/min
Injection size:
20 µL
System suitability
Samples:
Mobile phase, Standard solution, and Degradation standard solution
[Note—Chromatograph the Mobile phase, and verify that no extraneous peaks are present. ]
[Note—The relative retention times for the degradation product and leuprolide are about 0.90 and 1.0, respectively. ]
Suitability requirements
Retention time:
41–49 min for leuprolide, Degradation standard solution
Resolution:
NLT 1.5 between leuprolide and the degradation product, Degradation standard solution
Tailing factor:
0.8–1.5, Standard solution
Relative standard deviation:
NMT 1.5% for leuprolide acetate, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of leuprolide (C59H84N16O12) in the portion of Leuprolide Acetate taken:
Result = [(rU/rS) × (CS/CU) × P × M × 100]/(100  AC
AC  WC)
WC)
| rU | = | = peak area of the Sample solution |
| rS | = | = peak area of the Standard solution |
| CS | = | = concentration of USP Leuprolide Acetate RS in the Standard solution (µg/mL) |
| CU | = | = concentration of Leuprolide Acetate in the Sample solution (µg/mL) |
| P | = | = designated purity of USP Leuprolide Acetate RS (%) |
| M | = | = (100 |
| AC | = | = acetic acid content (%) |
| WC | = | = water content (%) |
Acceptance criteria:
97.0%–103.0% on the anhydrous and acetic acid-free basis
OTHER COMPONENTS
• Content of Acetic Acid
Diluent:
Methanol, adjusted with phosphoric acid to a pH of 2.5
Standard solution:
Pipet 2.0 mL of glacial acetic acid into a 100-mL volumetric flask, dilute with Diluent to volume, and mix. Transfer 4.0 mL of the solution to a 100-mL volumetric flask, dilute with Diluent to volume, and mix. Transfer 10.0 mL of this solution to a 100-mL volumetric flask, dilute with Diluent to volume, and mix to obtain a solution having a known concentration of about 0.08 mg/mL.
Sample solution:
Transfer about 100 mg of Leuprolide Acetate, accurately weighed, to a 100-mL volumetric flask, and dissolve in and dilute with Diluent to volume.
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.53-mm × 30-m fused-silica capillary column that contains a 1.2-µm film of phase G35
Temperature
Column:
100
Injection port:
200
Detector:
250
Carrier gas:
Helium
Flow rate:
10 mL/min
Injection size:
1.0 µL
Injection type:
Splitless mode
System suitability
Samples:
Diluent and Standard solution
Suitability requirements
Blank:
Chromatograph the Diluent, and verify that there are no interfering peaks.
Column efficiency:
NLT 15,000 theoretical plates, Standard solution
Tailing factor:
0.8–1.5, Standard solution
Relative standard deviation:
NMT 2.0% for glacial acetic acid, for replicate injections of the Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of acetic acid (C2H4O2) in the portion of Leuprolide Acetate taken:
Result = (rU/rS) × (839.2/WU)
| rU | = | = peak area of the Sample solution |
| rS | = | = peak area of the Standard solution |
| WU | = | = weight of Leuprolide Acetate taken to prepare the Sample solution (mg) |
Acceptance criteria:
4.7%–9.0%
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.3%
:
NMT 0.3%
• Chromatographic Purity
Solution A:
15.2 mg/mL of triethylamine in water. Adjust with phosphoric acid to a pH of 3.0 before final dilution.
Solution B:
Acetonitrile and n-propyl alcohol (3:2)
Mobile phase:
Solution A and Solution B (17:3)
Standard stock solution:
1 mg/mL of USP Leuprolide Acetate RS in Mobile phase
Standard solution:
Dilute 1.0 mL of the Standard stock solution with Mobile phase to 100.0 mL.
Degradation standard solution:
Dilute 5 mL of Standard stock solution with water to 50.0 mL. Transfer 5 mL of the solution to a scintillation vial. Add 100 µL of 1 N sodium hydroxide solution, tightly cap, and shake vigorously. Place in an oven at 100 for 60 min. Remove, allow to cool, add 50 µL of 1 M phosphoric acid, recap, and shake vigorously to mix.
for 60 min. Remove, allow to cool, add 50 µL of 1 M phosphoric acid, recap, and shake vigorously to mix.
Sample solution:
Transfer about 100 mg of Leuprolide Acetate to a 100-mL volumetric flask, and dissolve in and dilute with Mobile phase to volume.
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 10-cm; 3-µm packing L1
Flow rate:
1–1.5 mL/min
Injection size:
20 µL
System suitability
Samples:
Mobile phase, Standard solution, Degradation standard solution, and Sample solution
[Note—Chromatograph the Mobile phase, and verify that no extraneous peaks are present. ]
Suitability requirements
Retention time:
41–49 min for leuprolide, Degradation standard solution
Resolution:
NLT 1.5 between leuprolide and the degradation product, Degradation standard solution
Tailing factor:
0.8–1.5, Standard solution
Relative standard deviation:
NMT 1.5% for leuprolide acetate, Standard solution
Analysis
Samples:
Standard solution and Sample solution
[Note—Record the chromatograms for 90 min. ]
Calculate the percentage of each impurity in the portion of leuprolide acetate [C59H84N16O12·(C2H4O2)n] taken:
Result = (rU/rS) × (WS/WU) × P × M × 0.01
| rU | = | = peak response for each impurity from the Sample solution |
| rS | = | = peak response of leuprolide from the Standard stock solution |
| WS | = | = weight of USP Leuprolide Acetate RS in the Standard stock solution (mg) |
| WU | = | = weight of Leuprolide Acetate in the Sample solution (mg) |
| P | = | = designated purity of USP Leuprolide Acetate RS (%) |
| M | = | = (100 |
Acceptance criteria:
See Table 1.
Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Acetyl-leuprolide | 1.5 | 1.0 |
| d-His-leuprolide | 0.9 | 0.5 |
| l-Leu6-leuprolide | 1.2 | 0.5 |
| d-Ser-leuprolide | 0.8 | 0.5 |
| Leuprolide | 1.0 | — |
| Any other impurity | — | 0.5 |
| Total impurities | — | 2.5 |
SPECIFIC TESTS
• Amino Acid Content
[Note—Use a suitable, validated procedure (see Biotechnology-Derived Articles—Amino Acid Analysis  1052
1052 ). ]
). ]
Standard solutions:
Prepare a solution having known equimolar amounts of l-alanine, l-arginine, l-aspartic acid, l-glutamic acid, glycine, l-histidine, l-isoleucine, l-leucine, l-lysine, l-methionine, l-phenylalanine, l-proline, l-serine, l-threonine, l-tyrosine, and l-valine with half the equimolar amount of l-cystine. For the validation of the method, an appropriate internal standard, such as norleucine, is used. Prepare a separate, equimolar solution of l-tryptophan.
Sample solution:
Transfer 64 mg of Leuprolide Acetate to a suitable vessel. Dissolve in 1.0 mL of water. Transfer 0.10 mL of this solution to a vacuum hydrolysis tube. Add 2.0 mL of 6 N hydrochloric acid, evacuate the tube, and heat for 16 h at 120 . Transfer 0.10 mL of the hydrolysate so obtained to a suitable vessel, add 1 mL of water, and lyophilize. Dissolve in and dilute to a suitable volume in a buffer solution suitable for amino acid analysis.
. Transfer 0.10 mL of the hydrolysate so obtained to a suitable vessel, add 1 mL of water, and lyophilize. Dissolve in and dilute to a suitable volume in a buffer solution suitable for amino acid analysis.
Analysis:
Inject equal volumes of the Standard solution and Sample solution into the amino acid analyzer, and record and measure the responses for each amino acid peak. Express the content of each amino acid in moles.
Calculate the relative proportions of the amino acids in the Sample solution, taking one-seventh of the sum of the number of moles of histidine, glutamic acid, leucine, proline, tyrosine, and arginine as equal to one.
Acceptance criteria:
0.85–1.1 moles each of glutamic acid, proline, tyrosine, histidine, and arginine per mole of Leuprolide Acetate; 1.8–2.2 moles of leucine per mole of Leuprolide Acetate; serine and tryptophan are also present.
• Optical Rotation, Specific Rotation  781S
781S
Sample solution:
10 mg/mL, in 1% acetic acid
Acceptance criteria:
 38.0
38.0 to
to  42.0
42.0 expressed on an anhydrous, acetic acid-free basis
expressed on an anhydrous, acetic acid-free basis
• Water Determination, Method Ic  921
921 :
NMT 8.0%
:
NMT 8.0%
• Bacterial Endotoxins Test  85
85 :
It contains NMT 166.7 USP Endotoxin Units/mg of leuprolide acetate.
:
It contains NMT 166.7 USP Endotoxin Units/mg of leuprolide acetate.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers. Store at a temperature not higher than 30 .
.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Thomas A. Sigambris, M.S.
Scientific Liaison 1-301-998-6789 |
(BIO12010) Monographs - Biologics and Biotechnology 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3652
Pharmacopeial Forum: Volume No. 37(1)