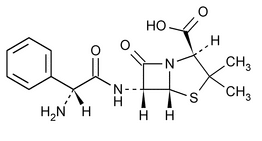
Ampicillin
C16H19N3O4S (anhydrous) 349.41
4-Thia-1-azabicyclo[3.2.0]heptane-2 carboxylic acid, [6-(aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-, [2S-[2 ,5
,5 ,6
,6 (S*)]]-;
(S*)]]-;
(2S,5R,6R)-6-[(R)-2-Amino-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid

 [69-53-4].
[69-53-4].
Trihydrate 403.46

 [7177-48-2].
[7177-48-2].
(am'' pi sil' in).
C16H19N3O4S (anhydrous) 349.41
4-Thia-1-azabicyclo[3.2.0]heptane-2 carboxylic acid, [6-(aminophenylacetyl)amino]-3,3-dimethyl-7-oxo-, [2S-[2
(2S,5R,6R)-6-[(R)-2-Amino-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid
Trihydrate 403.46
DEFINITION
Ampicillin is anhydrous or contains three molecules of water of hydration. It contains NLT 900 µg and NMT 1050 µg of C16H19N3O4S per mg, calculated on the anhydrous basis.
IDENTIFICATION
• Infrared Absorption  197K
197K :
Except that where the specimen under test is the trihydrate, both it and the USP Ampicillin Trihydrate RS are undried.
:
Except that where the specimen under test is the trihydrate, both it and the USP Ampicillin Trihydrate RS are undried.
ASSAY
• Procedure
[Note—The Standard solution and the Sample solution should be analyzed immediately after preparation. ]
Solution A:
6.54 mg/mL of monobasic potassium phosphate and 0.34 mg/mL of dibasic potassium phosphate, adjusted with 1 N sodium hydroxide or 1 N phosphoric acid to a pH of 5.5 before final dilution
Solution B:
Acetonitrile and Solution A (2:23)
Solution C:
Acetonitrile and Solution A (3:7)
Mobile phase:
See gradient table below.
| Time (min) | Solution B (%) | Solution C (%) |
|---|---|---|
| 0 | 100 | 0 |
| 6 | 100 | 0 |
| 15 | 0 | 100 |
| 16 | 0 | 100 |
| 18 | 100 | 0 |
| 20 | 100 | 0 |
Solution D:
46.3 mg/mL of monobasic potassium phosphate and 27.8 mg/mL of dibasic potassium phosphate, adjusted with 1 N sodium hydroxide or 1 N phosphoric acid to a pH of 6.5 before final dilution
System suitability solution:
0.5 mg/mL of USP Ampicillin RS and 0.1 mg/mL of USP Amoxicillin RS in acetonitrile, water, and Solution D (4:91:5). [Note—Dissolve first in a mixture of acetonitrile, water, and Solution D (4:30:5), sonicating if necessary, and dilute with water to volume. ]
Standard solution:
0.5 mg/mL of USP Ampicillin RS in acetonitrile, water, and Solution D (4:91:5). [Note—Dissolve first in a mixture of acetonitrile, water, and Solution D (4:30:5), sonicating if necessary, and dilute with water to volume. ]
Sample solution:
0.5 mg/mL of Ampicillin in acetonitrile, water, and Solution D (4:91:5). [Note—Dissolve first in a mixture of acetonitrile, water, and Solution D (4:30:5), sonicating if necessary, and dilute with water to volume. ]
Chromatographic system
Mode:
LC
Detector:
UV 230 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Flow rate:
1.5 mL/min
Injection size:
20 µL
System suitability
Samples:
System suitability solution and Standard solution
Suitability requirements
Resolution:
NLT 10 between the ampicillin and amoxicillin peaks, System suitability solution
Tailing factor:
NMT 1.4, System suitability solution
Relative standard deviation:
NMT 2.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the quantity, in µg, of C16H19N3O4S in each mg of Ampicillin taken:
Result = (rU/rS) × (CS/CU) × P
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Ampicillin RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of Ampicillin in the Sample solution (mg/mL) |
| P | = | = potency of USP Ampicillin RS (µg/mg) |
Acceptance criteria:
900 µg–1050 µg on the anhydrous basis
IMPURITIES
Organic Impurities
• Procedure 1
[Note—The Standard solution and the Sample solution should be analyzed immediately after preparation. ]
Solution A, Solution B, Solution C, Mobile phase, Solution D, System suitability solution, Sample solution, and Chromatographic system:
Prepare as directed in the Assay.
Standard stock solution:
Prepare as directed for Standard solution in the Assay.
Standard solution:
0.005 mg/mL of ampicillin in Solution D and water (1:19) from Standard stock solution. [Note—Transfer an aliquot of the Standard stock solution to a suitable volumetric flask, add Solution D, using about 5% of the final volume, dilute with water to volume. ]
Sensitivity solution:
0.0005 mg/mL of ampicillin in Solution D and water (1:19) from Standard solution. [Note—Transfer an aliquot of the Standard solution to a suitable volumetric flask, add Solution D, using about 5% of the final volume, and dilute with water to volume. ]
System suitability
Samples:
Sensitivity solution, System suitability solution, and Standard solution
Suitability requirements
Signal-to-noise ratio:
NLT 3, Sensitivity solution
Resolution:
NLT 10 between ampicillin and amoxicillin, System suitability solution
Tailing factor:
NMT 1.4, System suitability solution
Relative standard deviation:
NMT 10.0%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Ampicillin taken:
Result = (rU/rS) × (CS/CU) × P × F × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of ampicillin from the Standard solution |
| CS | = | = concentration of USP Ampicillin RS in the Standard solution (mg/mL) |
| CU | = | = nominal concentration of Ampicillin in the Sample solution (mg/mL) |
| P | = | = potency of USP Ampicillin RS (µg/mg) |
| F | = | = conversion factor, 0.001 mg/µg |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 3.0%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| d-Phenylglycinea | 0.27 | 0.5 |
| 6-Aminopenicillanic acidb | 0.31 | 0.5 |
| Ampicilloic acidc | 0.45 | 1.0 |
| Ampicillin thiazepine analogd | 0.65 | 0.3 |
| Ampicillin | 1.0 | — |
| Ampicillin rearrangement product (isomer 1)e | 1.8 | 0.4 |
| Ampicillin rearrangement product (isomer 2)e | 2.0 | 0.3 |
| Ampicillin oligomer 2f | 2.2 | 0.6 |
| d-Phenylglycylampicilling | 2.5 | 0.8 |
| Ampicillin oligomer 1 (dimer)h | 2.6 | 1.0 |
| Ampicillin oligomer 1 (trimer)i | 2.9 | 0.4 |
| Any individual unspecified impurity | — | 0.25 |
|
a
(R)-2-Amino-2-phenylacetic acid.
b
(2S,5R,6R)-6-Amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
c
(4S)-2-{[(R)-2-Amino-2-phenylacetamido](carboxy)methyl}-5,5-dimethylthiazolidine-4-carboxylic acid.
d
(S)-6-[(R)-2-Amino-2-phenylacetamido]-2,2-dimethyl-7-oxo-2,3,4,7-tetrahydro-1,4-thiazepine-3-carboxylic acid.
e
(4S)-2-(3,6-Dioxo-5-phenylpiperazin-2-yl)-5,5-dimethylthiazolidine-4-carboxylic acid.
f
(4S)-2-{1-[(R)-2-Amino-2-phenylacetamido]-2-[(1R)-2-{carboxy[(4S)-4-carboxy-5,5-dimethylthiazolidin-2-yl]methylamino}-2-oxo-1-phenylethylamino]-2-oxoethyl}-5,5-dimethylthiazolidine-4-carboxylic acid.
g
(2S,5R,6R)-6-{(R)-2-[(R)-2-Amino-2-phenylacetamido]-2-phenylacetamido}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
h
(2S,5R,6R)-6-[(2R)-2-{2-[(R)-2-Amino-2-phenylacetamido]-2-[(4S)-4-carboxy-5,5-dimethylthiazolidin-2-yl]acetamido}-2-phenylacetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
i
(4S,4'S)-2,2'-{(1R,7R,13R)-1-Amino-14-[(2S,5R,6R)-2-carboxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptan-6-ylamino]-2,5,8,11,14-pentaoxo-1,7,13-triphenyl-3,6,9,12-tetraazatetradecane-4,10-diyl}bis(5,5-dimethylthiazolidine-4-carboxylic acid).
|
||
• Procedure 2: Dimethylaniline  223
223 :
Meets the requirements
:
Meets the requirements
SPECIFIC TESTS
• Sterility Tests  71
71 :
Where the label states that Ampicillin is sterile, it meets the requirements when tested as directed for Test for Sterility of the Product to be Examined, Membrane Filtration, except to dissolve 6 g in 800 mL of Fluid D containing sufficient sterile penicillinase to inactivate the ampicillin and to swirl the vessel until solution is complete before filtering.
:
Where the label states that Ampicillin is sterile, it meets the requirements when tested as directed for Test for Sterility of the Product to be Examined, Membrane Filtration, except to dissolve 6 g in 800 mL of Fluid D containing sufficient sterile penicillinase to inactivate the ampicillin and to swirl the vessel until solution is complete before filtering.
• Crystallinity  695
695 :
Meets the requirements
:
Meets the requirements
• Water Determination, Method I  921
921 :
NMT 2.0% where it is labeled as Ampicillin (anhydrous); between 12.0% and 15.0% where it is labeled as Ampicillin (trihydrate)
:
NMT 2.0% where it is labeled as Ampicillin (anhydrous); between 12.0% and 15.0% where it is labeled as Ampicillin (trihydrate)
• Bacterial Endotoxins Test  85
85 :
Where the label states that Ampicillin is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.15 USP Endotoxin Unit/mg of ampicillin.
:
Where the label states that Ampicillin is sterile or must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.15 USP Endotoxin Unit/mg of ampicillin.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers.
• Labeling:
Label to indicate whether it is anhydrous or is the trihydrate. Where the quantity of ampicillin is indicated in the labeling of any preparation containing Ampicillin, this shall be understood to be in terms of anhydrous ampicillin (C16H19N3O4S). Where it is intended for use in preparing injectable dosage forms, the label states that it is the trihydrate and that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
• USP Reference Standards  11
11
USP Endotoxin RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2207
Pharmacopeial Forum: Volume No. 34(5) Page 1140