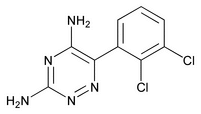
Lamotrigine
C9H7Cl2N5 256.09
1,2,4-Triazine-3,5-diamine, 6-(2,3-dichlorophenyl);
3,5-Diamino-6-(2,3-dichlorophenyl)-as-triazine

 [84057-84-1].
[84057-84-1].
(la moe' tri jeen).
C9H7Cl2N5 256.09
1,2,4-Triazine-3,5-diamine, 6-(2,3-dichlorophenyl);
3,5-Diamino-6-(2,3-dichlorophenyl)-as-triazine
DEFINITION
Lamotrigine contains NLT 98.0% and NMT 102.0% of C9H7Cl2N5, calculated on the dried basis.
IDENTIFICATION
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
ASSAY
• Procedure
Diluent:
Dilute 8.5 mL of hydrochloric acid with water to 1 L (0.1 M hydrochloric acid).
Buffer:
2.7 g/L of monobasic potassium phosphate in water
Solution A:
Triethylamine and Buffer (1:150). Adjust with phosphoric acid to a pH of 2.0.
Solution B:
Acetonitrile
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 76.5 | 23.5 |
| 4 | 76.5 | 23.5 |
| 14 | 20 | 80 |
| 15 | 76.5 | 23.5 |
| 19 | 76.5 | 23.5 |
Standard solution:
0.2 mg/mL of USP Lamotrigine RS prepared as follows. Transfer the required amount of USP Lamotrigine RS to a suitable volumetric flask, and add 5% of the final volume with methanol to facilitate dissolution. Dilute with Diluent to volume.
Sample solution:
0.2 mg/mL of Lamotrigine prepared as follows. Transfer the required amount of lamotrigine to a suitable volumetric flask, and add 5% of the final volume with methanol to facilitate dissolution. Dilute with Diluent to volume.
Chromatographic system
Mode:
LC
Detector:
UV 270 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
35
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 1.5
Relative standard deviation:
NMT 1.5%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of lamotrigine (C9H7Cl2N5) in the portion of Lamotrigine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Lamotrigine RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Lamotrigine in the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Heavy Metals, Method II  231
231 :
10 ppm
:
10 ppm
• Limit of Lamotrigine Related Compound B
Diluent, Solution A, and Sample solution:
Prepare as directed in the Assay.
Mobile phase:
Acetonitrile and Solution A (35:65)
System suitability stock solution:
0.2 mg/mL of USP Lamotrigine RS prepared as follows. Transfer the required amount of USP Lamotrigine RS to a suitable volumetric flask, and add 5% of the final volume with methanol to facilitate dissolution. Dilute with Diluent to volume.
Standard stock solution:
0.01 mg/mL of USP Lamotrigine Related Compound B RS prepared as follows. Transfer the required amount of USP Lamotrigine Related Compound B RS to a volumetric flask. Add 80% of the flask volume of methanol, and acidify with 1% of the flask volume of hydrochloric acid. Allow to cool, and dilute with methanol to volume. Dilute a portion of this solution with Diluent.
System suitability solution:
1 µg/mL of lamotrigine related compound B from the Standard stock solution in System suitability stock solution
Standard solution:
5 µg/mL of lamotrigine related compound B from the Standard stock solution in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 210 nm
Column:
4.6-mm × 15-cm; 5-µm packing L1
Column temperature:
35
Flow rate:
1 mL/min
Injection size:
10 µL
Run time:
2 times the retention time of lamotrigine related compound B
System suitability
Sample:
System suitability solution
[Note—Identify the peaks in the System suitability solution, taking into account that lamotrigine is unretained, eluting at or near the solvent front. ]
Suitability requirements
Tailing factor:
NMT 2.0 for the lamotrigine related compound B peak
Relative standard deviation:
NMT 5.0% for the lamotrigine related compound B peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of lamotrigine related compound B in the portion of Lamotrigine taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response for lamotrigine related compound B from the Sample solution |
| rS | = | = peak response for the lamotrigine related compound B from the Standard solution |
| CS | = | = concentration of USP Lamotrigine Related Compound B RS in the Standard solution (µg/mL) |
| CU | = | = concentration of Lamotrigine in the Sample solution (µg/mL) |
Acceptance criteria:
NMT 0.1% of lamotrigine related compound B. [Note—Lamotrigine related compound D, if present, will elute at a retention time of about 1.5 relative to lamotrigine related compound B. Disregard this peak as it is quantified in the test for Organic Impurities. ]
• Organic Impurities
Diluent, Buffer, Solution A, Solution B, Mobile phase, Sample solution, and Chromatographic system:
Proceed as directed in the Assay.
System suitability stock solution:
0.2 mg/mL of USP Lamotrigine RS prepared as follows. Transfer the required amount of USP Lamotrigine RS to a suitable volumetric flask, and add 5% of the final volume with methanol to facilitate dissolution. Dilute with Diluent to volume.
Impurities stock solution:
0.1 mg/mL of each of USP Lamotrigine Related Compound C RS and USP Lamotrigine Related Compound D RS prepared as follows. Transfer suitable quantities of the Reference Standards to a suitable volumetric flask. Add an amount of methanol equal to 80% of the flask volume, and acidify with 1% of the flask volume of hydrochloric acid. Allow to cool. Dilute with methanol to volume.
System suitability solution:
0.5 µg/mL each of lamotrigine related compound C and lamotrigine related compound D from Impurities stock solution in System suitability stock solution
System suitability
Suitability requirements
Resolution:
NLT 2.0 between lamotrigine and lamotrigine related compound C peaks
Analysis
Samples:
Diluent and Sample solution
[Note—Disregard any peak that may be present in the chromatogram of the Diluent injection. Disregard any peak due to lamotrigine related compound B, because it is quantified in the test for Limit of Lamotrigine Related Compound B. ]
Calculate the percentage of each impurity in the portion of Lamotrigine taken:
Result = (rU/rS) × (1/F) × 100
| rU | = | = peak response for each impurity from the Sample solution |
| rS | = | = peak response for the lamotrigine peak from the Sample solution |
| F | = | = relative response factor for each impurity from Table 2 |
Acceptance criteria:
See Table 2.
Table 2
| Name | Relative Retention Time |
Relative Response Factor |
Acceptance Criteria, NMT (%) |
|
|---|---|---|---|---|
| Lamotrigine | 1.0 | 1.0 | — | |
| Lamotrigine related compound Ca |
1.5 | 1.0 | 0.1 | |
| Lamotrigine related compound Bb,c |
3.2 | — | — | |
| Lamotrigine related compound Dd |
3.7 | 0.8 | 0.2 | |
| Any individual, unspecified impurity |
— | 1.0 | 0.1 | |
| Total impurities, excluding lamotrigine related compound B | — | — | 0.2 | |
|
a
3-Amino-6-(2,3-dichlorophenyl)-1,2,4-triazin-5(4H)-one.
b
2,3-Dichlorobenzoic acid.
c
Included only for identification.
d
N-[5-Amino-6-(2,3-dichlorophenyl)-1,2,4-triazin-3-yl]-2,3-dichlorobenzamide.
|
||||
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 for 3 h: it loses NMT 0.5% of its weight.
for 3 h: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers. Store at room temperature.
• USP Reference Standards  11
11
USP Lamotrigine RS
USP Lamotrigine Related Compound B RS
2,3-Dichlorobenzoic acid.
C7H4Cl2O2 191.01
2,3-Dichlorobenzoic acid.
C7H4Cl2O2 191.01
USP Lamotrigine Related Compound C RS
3-Amino-6-(2,3-dichlorophenyl)-1,2,4-triazin-5(4H)-one.
C9H6Cl2N4O 257.08
3-Amino-6-(2,3-dichlorophenyl)-1,2,4-triazin-5(4H)-one.
C9H6Cl2N4O 257.08
USP Lamotrigine Related Compound D RS
N-[5-Amino-6-(2,3-dichlorophenyl)-1,2,4-triazin-3-yl]-2,3-dichlorobenzamide.
C16H9Cl4N5O 429.09
N-[5-Amino-6-(2,3-dichlorophenyl)-1,2,4-triazin-3-yl]-2,3-dichlorobenzamide.
C16H9Cl4N5O 429.09
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Hariram Ramanathan, M.S.
Associate Scientific Liaison 1-301-816-8313 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3635
Pharmacopeial Forum: Volume No. 34(3) Page 617