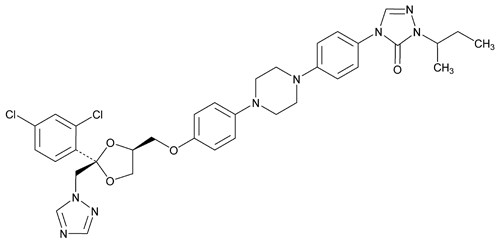
Itraconazole
(it'' ra kon' a zole).
3H-1,2,4-Triazol-3-one, 4-[4-[4-[4-[[2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-1-piperazinyl]phenyl]-2,4-dihydro-2-(1-methylpropyl)-.
(±)-1-sec-Butyl-4-[p-[4-[p-[[(2R*,4S*)-2-(2,4-dichlorophenyl)-2-(1H-1,2,4-triazol-1-ylmethyl)-1,3-dioxolan-4-yl]methoxy]phenyl]-1-piperazinyl]phenyl]-D2-1,2,4-triazolin-5-one
» Itraconazole contains not less than 98.5 percent and not more than 101.5 percent of C35H38Cl2N8O4, calculated on the dried basis.
Packaging and storage—
Preserve in tight, light-resistant containers, and store at room temperature.
Identification—
Angular rotation  781A
781A :
between
:
between  0.10
0.10 and +0.10
and +0.10 , measured at 20
, measured at 20 .
.
Test solution:
100 mg per mL, in methylene chloride.
Melting range  741
741 :
between 166
:
between 166 and 170
and 170 .
.
Loss on drying  731
731 —
Dry about 1 g at 105
—
Dry about 1 g at 105 for 4 hours: it loses not more than 0.5% of its weight.
for 4 hours: it loses not more than 0.5% of its weight.
Residue on ignition  281
281 :
not more than 0.1%, determined on 1.0 g.
:
not more than 0.1%, determined on 1.0 g.
Related compounds—
Solution A:
0.08 M tetrabutylammonium hydrogen sulfate.
Solution B:
acetonitrile.
Diluent—
Prepare a mixture of methanol and tetrahydrofuran (1:1).
Standard solution—
Dissolve an accurately weighed quantity of USP Itraconazole RS in Diluent, stepwise if necessary, to obtain a solution having a known concentration of about 0.05 mg per mL.
Resolution solution—
Dissolve suitable quantities of USP Itraconazole RS and USP Miconazole in RS in Diluent to obtain a solution having known concentrations of about 0.05 mg each per mL.
Test solution—
Dissolve an accurately weighed quantity of Itraconazole in Diluent to obtain a solution having a known concentration of about 10 mg per mL.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 225-nm detector and a 4.6-mm × 10-cm column that contains 3-µm packing L1. The flow rate is about 1.5 mL per minute. The column temperature is maintained at 30
)—
The liquid chromatograph is equipped with a 225-nm detector and a 4.6-mm × 10-cm column that contains 3-µm packing L1. The flow rate is about 1.5 mL per minute. The column temperature is maintained at 30 . The chromatograph is programmed as follows.
. The chromatograph is programmed as follows.
[note—Equilibrate the column for at least 30 minutes with acetonitrile at a flow rate of 1.5 mL per minute and then equilibrate at the initial eluent composition for at least 5 minutes. ] Chromatograph the Resolution solution, and record the peak responses as directed for Procedure: the resolution, R, between miconazole and itraconazole is not less than 2.0.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
|
|---|---|---|---|
| 0–20 | 80®50 | 20®50 | linear gradient |
| 20–25 | 50 | 50 | isocratic |
| 25–30 | 80 | 20 | equilibration |
Procedure—
Separately inject equal volumes (about 10 µL) of the Diluent, the Standard solution and the Test solution into the chromatograph and record the chromatograms. Calculate the percentage of each impurity in the portion of Itraconazole taken by the formula:
100(CS / CU)(rU / rS)
in which CS is the concentration, in mg per mL, of itraconazole in the Standard solution; CU is the concentration, in mg per mL, of Itraconazole in the Test solution; rU is the peak area for each impurity in the Test solution; and rS is the peak area for itraconazole in the Standard solution: not more than 0.5% of any specified impurity, as shown in Table 1, is found; and not more than 1.25% of total impurities is found. Disregard any peak observed in the Diluent and any peak less than 0.05%.
Table 1
| Common Name | Limit (%) | |
|---|---|---|
| 4-Methoxy derivative1 | 0.5 | |
| 4-Triazolyl isomer2 | 0.5 | |
| Propyl analog3 | 0.5 | |
| Isopropyl analog4 | 0.5 | |
| Epimer5 | 0.5 | |
| n-Butyl isomer6 | 0.5 | |
| Didioxolanyl analog7 | 0.5 | |
|
1
2-sec-Butyl-4-{4-[4-(4-methoxyphenyl)piperazin-1-yl]phenyl}-2H-1,2,4-triazol-3(4H)-one.
2
4-(4-{4-[4-({(2RS,4SR)-2-[(4H-1,2,4-Triazol-4-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-sec-butyl-2H-1,2,4-triazol-3(4H)-one.
3
4-(4-{4-[4-({(2RS,4SR)-2-[(1H-1,2,4-Triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-propyl-2H-1,2,4-triazol-3(4H)-one.
4
4-(4-{4-[4-({(2RS,4SR)-2-[(1H-1,2,4-Triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-isopropyl-2H-1,2,4-triazol-3(4H)-one.
5
4-(4-{4-[4-({(2RS,4RS)-2-[(1H-1,2,4-Triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-sec-butyl-2H-1,2,4-triazol-3(4H)-one.
6
4-(4-{4-[4-({(2RS,4SR)-2-[(1H-1,2,4-Triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-butyl-2H-1,2,4-triazol-3(4H)-one.
7
4-(4-{4-[4-({(2RS,4SR)-2-[(1H-1,2,4-Triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methoxy)phenyl]piperazin-1-yl}phenyl)-2-({(2RS,4SR)-2-[(1H-1,2,4-triazol-1-yl)methyl]-2-(2,4-dichlorophenyl)-1,3-dioxolan-4-yl}methyl)-2H-1,2,4-triazol-3(4H)-one.
|
||
Assay—
Diluent—
Prepare a mixture of methyl ethyl ketone and glacial acetic acid (7:1).
Procedure—
Dissolve about 0.3 g of Itraconazole, accurately weighed, in 70 ml of Diluent. Titrate with 0.1 M perchloric acid, determining the endpoint potentiometrically at the second inflection point. One mL of 0.1 M perchloric acid is equivalent to 35.3 mg of C35H38Cl2N8O4.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientific Liaison 1-301-816-8394 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3604
Pharmacopeial Forum: Volume No. 37(3)