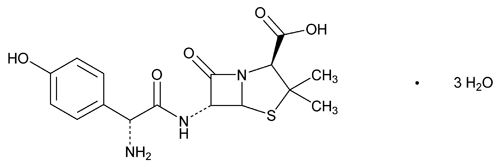
Amoxicillin
C16H19N3O5S·3H2O 419.45
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[amino(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-, trihydrate [2S-[2 ,5
,5 ,6
,6 (S*)]]-;
(S*)]]-;
(2S,5R,6R)-6-[(R)-(–)-2-Amino-2-(p-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate

 [61336-70-7].
[61336-70-7].
Anhydrous 365.41

 [26787-78-0].
[26787-78-0].
(a mox'' i sil' in).
C16H19N3O5S·3H2O 419.45
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid, 6-[[amino(4-hydroxyphenyl)acetyl]amino]-3,3-dimethyl-7-oxo-, trihydrate [2S-[2
(2S,5R,6R)-6-[(R)-(–)-2-Amino-2-(p-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid trihydrate
Anhydrous 365.41
DEFINITION
Amoxicillin contains NLT 900 µg and NMT 1050 µg of C16H19N3O5S per mg, calculated on the anhydrous basis.
IDENTIFICATION
ASSAY
• Procedure
Diluent:
6.8 g/L of monobasic potassium phosphate in water. Adjust with a 45% (w/w) solution of potassium hydroxide to a pH of 5.0 ± 0.1.
Mobile phase:
Acetonitrile and Diluent (1:24)
Standard solution:
1.2 mg/mL of USP Amoxicillin RS in Diluent. [Note—Use this solution within 6 h. ]
Sample solution:
1.2 mg/mL of Amoxicillin in Diluent. [Note—Use this solution within 6 h. ]
Chromatographic system
Mode:
LC
Detector:
UV 230 nm
Column:
4-mm × 25-cm; packing L1
Flow rate:
1.5 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 2.5
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the quantity, in µg/mg, of C16H19N3O5S in the portion of Amoxicillin taken:
Result = (rU/rS) × (CS/CU) × P
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Amoxicillin RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Sample solution (mg/mL) |
| P | = | = potency of amoxicillin in USP Amoxicillin RS (µg/mg) |
Acceptance criteria:
900–1050 µg of C16H19N3O5S per mg on the anhydrous basis
IMPURITIES
Organic Impurities
• Procedure
Solution A:
2.72 g/L of monobasic potassium phosphate. Adjust with 1 N potassium hydroxide or 20% phosphoric acid to a pH of 5.0 ± 0.1.
Solution B:
Methanol
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 97 | 3 |
| 10 | 97 | 3 |
| 22 | 75 | 25 |
| 26 | 97 | 3 |
Standard solution:
12.5 µg/mL of USP Amoxicillin RS in Solution A
System suitability solution:
12.5 µg/mL each of USP Amoxicillin Related Compound A RS and USP Amoxicillin Related Compound D RS in Solution A
Sample solution:
1.25 mg/mL of Amoxicillin in Solution A. [Note—Store this solution at 4 and use within 4 h. ]
and use within 4 h. ]
Chromatographic system
Mode:
LC
Detector:
UV 210 nm
Column:
4.6-mm × 10-cm; 5-µm packing L1
Column temperature:
40
Flow rate:
1.5 mL/min
Injection size:
10 µL
Autosampler temperature:
4
System suitability
Samples:
Standard solution and System suitability solution
Suitability requirements
[Note—Identify peaks by the relative retention times in Impurity Table 1. ]
Resolution:
NLT 1.5 between amoxicillin related compound A and the second peak for amoxicillin related compound D, System suitability solution
Relative standard deviation:
NMT 10%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Amoxicillin taken:
Result = (rU/rS) × (CS/CU) × F × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of amoxicillin from the Standard solution |
| CS | = | = concentration of USP Amoxicillin RS in the Standard solution (µg/mL) |
| CU | = | = nominal concentration of Amoxicillin in the Sample solution (mg/mL) |
| F | = | = unit conversion factor (0.001 mg/µg) |
Acceptance criteria
[Note—The reporting limit is 0.03% of the amoxicillin peak from the Standard solution. ]
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 5.0%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Amoxicillin related compound Ia (d-hydroxyphenylglycine) | 0.32 | 1.0 |
| Amoxicillin related compound Db,c (amoxicillin open ring) | 0.53 | 1.0 |
| 0.68 | 1.0 | |
| Amoxicillin related compound Ad (6-aminopenicillanic acid) | 0.78 | 0.5 |
| Amoxicillin related compound Be,f (l-amoxicillin) | 0.87 | — |
| Amoxicillin | 1.0 | — |
| Amoxicillin related compound Gg (d-hydroxyphenylglycylamoxicillin) | 2.9 | 1.0 |
| Amoxicillin related compound Eh,i (amoxicillin penilloic derivative) | 4.5 | 1.0 |
| Amoxicillin related compound Mj (N-(penicillan-6-yl) open ring amoxicillinamide) | 6.0 | 1.0 |
| Amoxicillin related compound Fe,k (phenylpyrazinediol) | 6.3 | — |
| Amoxicillin related compound Cl (amoxicillin rearrangement product) | 6.4 | 1.0 |
| Amoxicillin related compound Eh,i (amoxicillin penilloic derivative) | 6.7 | 1.0 |
| Amoxicillin related compound Jm (amoxicillin open ring dimer) | 8.8 | 1.0 |
| Amoxicillin related compound Ln (N-(penicillan-6-yl) amoxicillinamide) | 9.0 | 1.0 |
| Any unspecified individual impurity | — | 1.0 |
|
a
(R)-2-Amino-2-(4-hydroxyphenyl)acetic acid.
b
The chromatographic system resolves two penicilloic acids from each other.
c
(4S)-2-{[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido](carboxy)methyl}-5,5-dimethylthiazolidine-4-carboxylic acid.
d
(2S,5R,6R)-6-Amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
e
These compounds are listed for information only and are not to be reported.
f
(2S,5R,6R)-6-[(S)-2-Amino-2-(4-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
g
(2S,5R,6R)-6-{(R)-2-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-2-(4-hydroxyphenyl)acetamido}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
h
The chromatographic system resolves two penilloic acids from each other.
i
(4S)-2-{[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]methyl}-5,5-dimethylthiazolidine-4-carboxylic acid.
j
(2S,5R,6R)-6-(2-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-2-((4S)-4-carboxy-5,5-dimethylthiazolidin-2-yl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
k
3-(4-Hydroxyphenyl)pyrazin-2-ol.
l
(4S)-2-[5-(4-Hydroxyphenyl)-3,6-dioxopiperazin-2-yl]-5,5-dimethylthiazolidine-4-carboxylic acid.
m
(2S,5R,6R)-6-((2R)-2-{2-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-2-[(4S)-4-carboxy-5,5-dimethylthiazolidin-2-yl]acetamido}-2-(4-hydroxyphenyl)acetamido)-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
n
(2S,5R,6R)-6-{(2S,5R,6R)-6-[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxamido}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid.
|
||
SPECIFIC TESTS
• Crystallinity  695
695 :
Meets the requirements
:
Meets the requirements
• Dimethylaniline  223
223 :
Meets the requirement
:
Meets the requirement
• Water Determination, Method I  921
921 :
11.5%–14.5%
:
11.5%–14.5%
• Sterility Tests  71
71 :
Where the label states that Amoxicillin is sterile, it meets the requirements when tested as directed in Test for Sterility of the Product to Be Examined, Direct Inoculation of the Culture Medium, except to use Fluid Thioglycollate Medium containing polysorbate 80 solution (5 mg/mL) and an amount of sterile penicillinase sufficient to inactivate the amoxicillin in each tube, to use Soybean–Casein Digest Medium containing polysorbate 80 solution (5 mg/mL) and an amount of sterile penicillinase sufficient to inactivate the amoxicillin in each tube, and to shake the tubes once daily.
:
Where the label states that Amoxicillin is sterile, it meets the requirements when tested as directed in Test for Sterility of the Product to Be Examined, Direct Inoculation of the Culture Medium, except to use Fluid Thioglycollate Medium containing polysorbate 80 solution (5 mg/mL) and an amount of sterile penicillinase sufficient to inactivate the amoxicillin in each tube, to use Soybean–Casein Digest Medium containing polysorbate 80 solution (5 mg/mL) and an amount of sterile penicillinase sufficient to inactivate the amoxicillin in each tube, and to shake the tubes once daily.
• Bacterial Endotoxins Test  85
85 :
Where the label states that Amoxicillin is sterile or Amoxicillin must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.25 USP Endotoxin Unit/mg of amoxicillin.
:
Where the label states that Amoxicillin is sterile or Amoxicillin must be subjected to further processing during the preparation of injectable dosage forms, it contains NMT 0.25 USP Endotoxin Unit/mg of amoxicillin.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, and store at controlled room temperature.
• Labeling:
Where it is intended for use in preparing injectable dosage forms, the label states that it is intended for veterinary use only and that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms. Label all other Amoxicillin to indicate that it is to be used in the manufacture of nonparenteral drugs only.
• USP Reference Standards  11
11
USP Amoxicillin Related Compound A RS 
(2S,5R,6R)-6-Amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 6-aminopenicillanic acid.
C8H12N2O3S 216.26

(2S,5R,6R)-6-Amino-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid; 6-aminopenicillanic acid.
C8H12N2O3S 216.26
USP Amoxicillin Related Compound D RS 
(4S)-2-{[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido](carboxy)methyl}-5,5-dimethylthiazolidine-4-carboxylic acid; amoxicillin open ring.
C16H21N3O6S 383.42

(4S)-2-{[(R)-2-Amino-2-(4-hydroxyphenyl)acetamido](carboxy)methyl}-5,5-dimethylthiazolidine-4-carboxylic acid; amoxicillin open ring.
C16H21N3O6S 383.42
USP Endotoxin RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ahalya Wise, M.S.
Senior Scientific Liaison 1-301-816-8161 |
(SM12010) Monographs - Small Molecules 1 |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Radhakrishna S Tirumalai, Ph.D.
Principal Scientific Liaison 1-301-816-8339 |
(GCM2010) General Chapters - Microbiology | |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 2195
Pharmacopeial Forum: Volume No. 36(1) Page 66