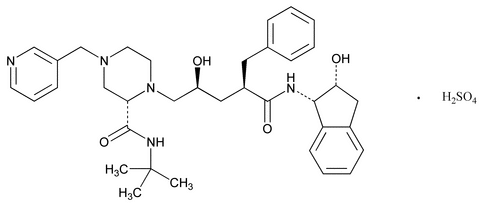
Indinavir Sulfate
C36H47N5O4·H2SO4 711.87
d-erythro-Pentonamide, 2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-[2-[[(1,1-dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-, [1(1S,2R),5(S)]-, sulfate (1:1) (salt);
( R,
R, S,2S)-
S,2S)- -Benzyl-2-(tert-butylcarbamoyl)-
-Benzyl-2-(tert-butylcarbamoyl)- -hydroxy-N-[(1S,2R)-2-hydroxy-1-indanyl]-4-(3-pyridylmethyl)-1-piperazinevaleramide sulfate (1:1) (salt)
-hydroxy-N-[(1S,2R)-2-hydroxy-1-indanyl]-4-(3-pyridylmethyl)-1-piperazinevaleramide sulfate (1:1) (salt)


 [157810-81-6].
[157810-81-6].
(in din' a vir sul' fate).
C36H47N5O4·H2SO4 711.87
d-erythro-Pentonamide, 2,3,5-trideoxy-N-(2,3-dihydro-2-hydroxy-1H-inden-1-yl)-5-[2-[[(1,1-dimethylethyl)amino]carbonyl]-4-(3-pyridinylmethyl)-1-piperazinyl]-2-(phenylmethyl)-, [1(1S,2R),5(S)]-, sulfate (1:1) (salt);
(
DEFINITION
Indinavir Sulfate contains NLT 98.5% and NMT 101.5% of C36H47N5O4·H2SO4, calculated on the anhydrous, solvent-free basis.
IDENTIFICATION
• A. Infrared Absorption  197M
197M :
Maxima at about 3.0–3.1, 5.9, 6.2, and 13.6 µm
:
Maxima at about 3.0–3.1, 5.9, 6.2, and 13.6 µm
• B.
The retention time of the major peak of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• C. Identification Tests—General, Sulfate  191
191 :
Meets the requirements
:
Meets the requirements
Sample solution:
A solution of 10 mg/mL in water
ASSAY
• Procedure
Solution A:
Dibutyl ammonium phosphate and water (1:50). Adjust with sodium hydroxide TS to a pH of 6.5 ± 0.5.
Mobile phase:
Acetonitrile and Solution A (9:11)
Standard solution:
0.5 mg/mL of USP Indinavir RS in Mobile phase
Sample solution:
0.6 mg/mL of Indinavir Sulfate in Mobile phase
Chromatographic system
Mode:
LC
Detector:
UV 260 nm
Column:
4.6-mm × 25-cm; 5-µm packing L7
Column temperature:
40
Flow rate:
1 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Column efficiency:
NLT 4000 theoretical plates
Tailing factor:
Less than 2.0
Relative standard deviation:
NMT 1.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C36H47N5O4·H2SO4 in the portion taken:
Result = (rU/rS) × (CS/CU) × (Mr1/Mr2) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Indinavir RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Indinavir Sulfate in the Sample solution (mg/mL) |
| Mr1 | = | = molecular weight of indinavir sulfate, 711.87 |
| Mr2 | = | = molecular weight of indinavir, 613.79 |
Acceptance criteria:
98.5%–101.5% on the anhydrous, solvent-free basis
OTHER COMPONENTS
• Procedure 1: Content of Sulfate
Solution A:
Methanol and formaldehyde (1000:0.3)
Diluent:
Solution A and water (1:1)
Sample solution:
6.25 mg/mL of Indinavir Sulfate in Diluent
Analysis:
Titrate with 0.1 M lead perchlorate VS, using a lead-specific electrode in conjunction with a suitable reference electrode. Each mL of 0.1 M lead perchlorate VS is equivalent to 9.604 mg of sulfate.
Acceptance criteria:
13.2%–14.4%, calculated on the anhydrous and solvent-free basis
• Procedure 2: Content of Alcohol
Standard solution:
0.001 mL/mL of dehydrated alcohol, in water. [Note—Dehydrated alcohol is at 20 . ]
. ]
Sample solution:
4 mg/mL of Indinavir Sulfate in water
Chromatographic system
Mode:
GC
Detector:
Flame ionization
Column:
0.53-mm × 30-m capillary column with a 1.0-µm film of phase G14
Temperature
Column:
35
Injector:
140
Detector:
220
[Note—At the end of each 5-min isothermal run, increase the oven temperature to 200 before adjusting the column temperature to 35
before adjusting the column temperature to 35 for the next injection. ]
for the next injection. ]
Flow rate:
10 mL/min
Carrier gas:
Helium
Injection size:
0.1 µL
System suitability
Sample:
Standard solution
Suitability requirements
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of alcohol in the portion of Indinavir Sulfate taken:
Result = (rU/rS) × (CS/CU) × D × 100
| rU | = | = peak area from the Sample solution |
| rS | = | = peak area from the Standard solution |
| CS | = | = concentration of dehydrated alcohol in the Standard solution (mL/mL) |
| CU | = | = concentration of Indinavir Sulfate in the Sample solution (mg/mL) |
| D | = | = density of alcohol at 20 |
Acceptance criteria:
5.0%–8.0%
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Heavy Metals  231
231
Standard solution:
Transfer 2 mL of Standard Lead Solution (10 µg/mL) to a 50-mL color-comparison tube, and dilute with water to 25 mL. Using a pH meter or short-range pH indicator paper as an external indicator, adjust with 1 N acetic acid or 6 N ammonium hydroxide to a pH between 3.0 and 4.0. Dilute with water to 40 mL.
Sample solution:
Transfer 2 g of Indinavir Sulfate to a 50-mL color-comparison tube, and dissolve in 25 mL of water. Using a pH meter or a suitable short-range pH indicator paper as an external indicator, adjust with 1 N acetic acid or 6 N ammonium hydroxide to a pH between 3.0 and 4.0. Dilute with water to 40 mL.
Blank solution:
To a 50-mL color-comparison tube, add 25 mL of water. Using a pH meter or a suitable short-range pH indicator paper as an external indicator, adjust with 1 N acetic acid or 6 N ammonium hydroxide to a pH between 3.0 and 4.0. Dilute with water to 40 mL.
Analysis:
To each tube, add 10 mL of hydrogen sulfide TS, allow to stand for 5 min, and view downward over a white surface.
Acceptance criteria:
The color of the Sample solution is not darker than that of the Standard solution, and the intensity of the color of the Blank solution is less than or equal to the intensity of that of the Sample solution.
Organic Impurities
• Procedure
Solution A:
0.27 g/L of monobasic potassium phosphate and 1.395 g/L of dibasic potassium phosphate, in water
Solution B:
Acetronitrile
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 80 | 20 |
| 40 | 30 | 70 |
| 45 | 30 | 70 |
| 47 | 80 | 20 |
| 52 | 80 | 20 |
Diluent:
Solution A and Solution B (1:1)
System suitability solution:
0.4 mg/mL of USP Indinavir System Suitability RS in Diluent
Sample solution:
0.5 mg/mL of Indinavir Sulfate in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 220 nm
Column:
4.6-mm × 25-cm; 5-µm packing L1
Flow rate:
1 mL/min
Injection size:
20 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 1.8 between indinavir and indinavir related compound C
Tailing factor:
More than 0.95 and less than 2.0, determined from the indinavir peak
Analysis
Sample:
Sample solution
Calculate the percentage of each impurity in the portion of Indinavir Sulfate taken:
Result = (rU/rT) × 100
| rU | = | = peak area response for each impurity |
| rT | = | = sum of the responses of all the peaks |
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 0.5%
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| cis-Aminoindanola | 0.18 | 0.1 |
| Desnicotinyl indinavirb | 0.80 | 0.1 |
| threo-Indinavirc | 0.98 | 0.1 |
| Indinavir lactoned | 1.14 | 0.1 |
| Diindanyl indinavire | 1.30 | 0.1 |
|
a
(1S,2R)-1-Aminoindan-2-ol.
b
(S)-1-{(2S,4R)-4-Benzyl-2-hydroxy-5-[(1S,2R)-2-hydroxyindan-1-ylamino]-5-oxopentyl}-N-tert-butylpiperazine-2-carboxamide.
c
(S)-1-{(2R,4R)-4-Benzyl-2-hydroxy-5-[(1S,2R)-2-hydroxyindan-1-ylamino]-5-oxopentyl}-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide.
d
(S)-1-{[(2S,4R)-4-Benzyl-5-oxotetrahydrofuran-2-yl]methyl}-N-tert-butyl-4-(pyridin-3-ylmethyl)piperazine-2-carboxamide.
e
(2R,2'R,4S,4'S)-5,5'-[(S)-2-(tert-Butylcarbamoyl)piperazine-1,4-diyl]bis{2-benzyl-4-hydroxy-N-[(1S,2R)-2-hydroxy-2,3-dihydro-1H-inden-1-yl]pentanamide}.
|
||
SPECIFIC TESTS
• Optical Rotation, Specific Rotation  781S
781S :
+122
:
+122 to +129
to +129 , at 365 nm, determined on the anhydrous, solvent-free basis
, at 365 nm, determined on the anhydrous, solvent-free basis
Sample solution:
10 mg/mL in water
• Water Determination, Method I  921
921 :
NMT 1.5%, using 0.25 g
:
NMT 1.5%, using 0.25 g
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight containers, protected from moisture. Store at 25 , excursions permitted between 15
, excursions permitted between 15 and 30
and 30 .
.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Behnam Davani, Ph.D., M.B.A.
Senior Scientific Liaison 1-301-816-8394 |
(SM12010) Monographs - Small Molecules 1 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3489
Pharmacopeial Forum: Volume No. 36(2) Page 406