Hypromellose
Cellulose, 2-hydroxypropyl methyl ether;
Cellulose hydroxypropyl methyl ether

 [9004-65-3].
[9004-65-3].
(hye proe' me lose).
| Attribute | JP | EP | USP |
|---|---|---|---|
| Definition | + | + | + |
| Labeling | + | + | + |
| Identification (A) | + | + | + |
| Identification (B) | + | + | + |
| Identification (C) | + | + | + |
| Identification (D) | + | + | + |
| Identification (E) | + | + | + |
| Viscosity, Method 1 | + | + | + |
| Viscosity, Method 2 | + | + | + |
| pH | + | + | + |
| Heavy Metals | + | + | + |
| Loss on Drying | + | + | + |
| Residue on Ignition | + | + | + |
| Assay | + | + | + |
Legend:
+ will adopt and implement;  will not stipulate
will not stipulate
Nonharmonized attributes:
Packaging and Storage
Specific local attributes:
Appearance of solution (EP), Description (JP), Limit of glyoxal (EP)
Cellulose, 2-hydroxypropyl methyl ether;
Cellulose hydroxypropyl methyl ether
DEFINITION
Hypromellose is a methyl and hydroxypropyl mixed ether of cellulose. It contains, calculated on the dried basis, methoxy (–OCH3: 31.03) and hydroxypropoxy (–OC3H6OH: 75.09) groups conforming to the limits for the types of Hypromellose (hydroxypropyl methylcellulose) set forth in the table below.
| Methoxy (%) | Hydroxypropoxy (%) | |||
|---|---|---|---|---|
| Substitution Type | Min. | Max. | Min. | Max. |
| 1828 | 16.5 | 20.0 | 23.0 | 32.0 |
| 2208 | 19.0 | 24.0 | 4.0 | 12.0 |
| 2906 | 27.0 | 30.0 | 4.0 | 7.5 |
| 2910 | 28.0 | 30.0 | 7.0 | 12.0 |
IDENTIFICATION
• A. Procedure
Sample:
1 g
Analysis:
Gently add the Sample to the top of 100 mL of water in a beaker, and allow to disperse over the surface, tapping the top of the container to ensure an even dispersion of the substance. Allow the beaker to stand for 1–2 min.
Acceptance criteria:
The powdered material aggregates on the surface.
• B. Procedure
Sample:
1 g
Analysis:
Add the Sample to 100 mL of boiling water, and stir the mixture using a magnetic stirrer with a bar 25 mm long.
Acceptance criteria:
A slurry is formed, but the powdered material does not dissolve. Cool the slurry to 10 , and stir using a magnetic stirrer: the resulting liquid is a clear or slightly turbid solution with thickness dependent on the viscosity grade.
, and stir using a magnetic stirrer: the resulting liquid is a clear or slightly turbid solution with thickness dependent on the viscosity grade.
• C. Procedure
Solution A:
Sulfuric acid and water (9:1). [Note—Carefully add sulfuric acid to water. ]
Sample solution:
0.1 mL of the solution prepared for Identification test B
Analysis:
To the Sample solution, add 9 mL of Solution A, and shake. Heat in a water bath for exactly 3 min, immediately cool in an ice bath, and add carefully 0.6 mL of ninhydrin TS. Shake, and allow to stand at 25 .
.
Acceptance criteria:
A red color develops at first that changes to purple within 100 min.
• D. Procedure
Sample solution:
2–3 mL of the solution prepared for Identification test B
Analysis:
Pour the Sample solution onto a glass slide as a thin film, and allow the water to evaporate.
Acceptance criteria:
A coherent, clear film forms on the glass slide.
• E. Procedure
Sample solution:
50 mL of the solution prepared in Identification test B
Analysis:
Add the Sample solution to exactly 50 mL of water in a beaker. Insert a thermometer into the solution. Stir the solution on a magnetic stirrer/hot plate, and begin heating at a rate of 2 to 5
to 5 /min. Determine the temperature at which a turbidity increase begins to occur, and designate this temperature as the flocculation temperature.
/min. Determine the temperature at which a turbidity increase begins to occur, and designate this temperature as the flocculation temperature.
Acceptance criteria:
The flocculation temperature is higher than 50 .
.
ASSAY
• Procedure
[Caution—Hydriodic acid and its reaction byproducts are highly toxic. Perform all steps in the preparation of the Standard solution and the Sample solution in a properly functioning hood. Specific safety practices to be followed are to be identified to the analyst performing this test.
]
Apparatus:
For the reaction vial, use a 5-mL pressure-tight serum vial, 50 mm in height, 20 mm in outside diameter, and 13 mm in inside diameter at the mouth. The vial is equipped with a pressure-tight septum having a polytetrafluoroethylene-faced butyl rubber and an airtight seal using an aluminum crimp or any sealing system that provides sufficient airtightness. Use a heater having a heating module that has a square-shape aluminum block with holes 20 mm in diameter and 32 mm in depth, into which the reaction vial fits. The heating module is also equipped with a magnetic stirrer capable of mixing the contents of the vial, or use a reciprocal shaker that performs a reciprocating motion of about 100 times/min.
Hydriodic acid:
Use a reagent having a typical concentration of HI of about 57%.
Internal standard solution:
30 mg/mL of n-octane ino-xylene
Standard solution:
Into a suitable serum vial, weigh between 60 and 100 mg of adipic acid, and add 2.0 mL of Hydriodic acid and 2.0 mL of Internal standard solution. Close the vial securely with a suitable septum stopper. Weigh the vial and contents, add between 15 µL and 22 µL of isopropyl iodide through the septum with a syringe, weigh again, and calculate the weight of isopropyl iodide added, by difference. Add 45 µL of methyl iodide similarly, weigh again, and calculate the weight of methyl iodide added, by difference. Shake the reaction vial well, and allow the layers to separate. Use the upper layer as the Standard solution.
Sample solution:
Transfer 0.065 g of dried Hypromellose to a 5-mL thick-walled reaction vial equipped with a pressure-tight septum-type closure, add between 60 and 100 mg of adipic acid, and pipet 2.0 mL of Internal standard solution into the vial. Cautiously pipet 2.0 mL of Hydriodic acid into the mixture, immediately cap the vial tightly, and weigh. Using the magnetic stirrer equipped in the heating module, or using a reciprocal shaker, mix the contents of the vial continuously, heating and maintaining the temperature of the contents at 130 ± 2 for 60 min. If a reciprocal shaker or magnetic stirrer cannot be used, shake the vial well by hand at 5-min intervals during the initial 30 min of the heating time. Allow the vial to cool, and weigh. If the weight loss is
for 60 min. If a reciprocal shaker or magnetic stirrer cannot be used, shake the vial well by hand at 5-min intervals during the initial 30 min of the heating time. Allow the vial to cool, and weigh. If the weight loss is 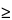 0.50% of the contents or there is evidence of a leak, discard the mixture, and prepare another Sample solution.
0.50% of the contents or there is evidence of a leak, discard the mixture, and prepare another Sample solution.
Chromatographic system
Mode:
GC
Detector:
Thermal conductivity or hydrogen flame-ionization
Column:
3- to 4-mm × 1.8- to 3-m glass column packed with 20% liquid phase G28 on 100- to 120-mesh support S1C that is not silanized
[Note—Use a column giving well-resolved peaks of methyl iodide, isopropyl iodide, and the internal standard, in that order. ]
Column temperature:
100
Carrier gas:
Use helium with the thermal conductivity detector; helium or nitrogen can be used for the hydrogen flame-ionization detector.
Flow rate:
With the Standard solution, adjust the flow rate so that the retention time of the internal standard is about 10 min.
Injection size:
1–2 µL
Analysis
Samples:
Upper layer of the Standard solution and the Sample solution
Calculate the percentage of –OCH3 in the portion of Hypromellose taken:
Result = 21.864 × (RUa/RSa) × (WSa/WU)
| RUa | = | = peak area ratio of methyl iodide to n-octane from the Sample solution |
| RSa | = | = peak area ratio of methyl iodide to n-octane from the Standard solution |
| WSa | = | = weight of methyl iodide in the Standard solution (mg) |
| WU | = | = weight of Hypromellose, calculated on the dried basis, taken for the Sample solution (mg) |
Calculate the percentage of –OC3H6OH in the portion of Hypromellose taken:
Result = 44.17 × (RUb/RSb) × (WSb/WU)
| RUb | = | = peak area ratio of isopropyl iodide to n-octane from the Sample solution |
| RSb | = | = peak area ratio of isopropyl iodide to n-octane from the Standard solution |
| WSb | = | = weight of isopropyl iodide in the Standard solution (mg) |
| WU | = | = weight of Hypromellose, calculated on the dried basis, taken for the Sample solution (mg) |
Acceptance criteria:
See the limits, calculated on the dried basis, in the table in the Definition.
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 1.5% on a 1.0-g sample
:
NMT 1.5% on a 1.0-g sample
• Heavy Metals, Method III  231
231 :
NMT 20 ppm
:
NMT 20 ppm
SPECIFIC TESTS
• pH  791
791 :
5.0–8.0, measured on the solution prepared in the tests for Viscosity at a temperature of 20 ± 2
:
5.0–8.0, measured on the solution prepared in the tests for Viscosity at a temperature of 20 ± 2 . Read the indicated pH value after the probe has been immersed for 5 ± 0.5 min.
. Read the indicated pH value after the probe has been immersed for 5 ± 0.5 min.
• Loss on Drying  731
731 :
Dry 1.0 g at 105
:
Dry 1.0 g at 105 for 1 h: it loses NMT 5.0% of its weight.
for 1 h: it loses NMT 5.0% of its weight.
• Viscosity  911
911
For hypromellose samples having a viscosity type of less than 600 mPa·s
Sample solution:
Transfer a quantity of Hypromellose equivalent to 4 g of solids, calculated on the dried basis, to a tared, wide-mouth centrifuge bottle. Add hot water to obtain a total weight of the sample and water of 200.0 g. Capping the bottle, stir by mechanical means at 400 ± 50 rpm for 10–20 min until the particles are thoroughly dispersed and wetted out. Scrape down the walls of the bottle with a spatula, if necessary, to ensure that there is no undissolved material on the sides of the bottle, and continue the stirring in a cooling water bath equilibrated at a temperature below 10 for another 20–40 min. Adjust the solution weight, if necessary, to 200.0 g, using cold water. Centrifuge the solution to expel any entrapped air. If any foam is present, remove with a spatula.
for another 20–40 min. Adjust the solution weight, if necessary, to 200.0 g, using cold water. Centrifuge the solution to expel any entrapped air. If any foam is present, remove with a spatula.
Analysis:
Determine the viscosity in a suitable viscosimeter of the Ubbelohde type as directed under Viscosity  911
911 .
.
Acceptance criteria:
80%–120% of the viscosity stated on the label
For hypromellose samples having a viscosity type of 600 mPa·s or higher
Sample solution:
Transfer a quantity of Hypromellose equivalent to 10 g of solids, calculated on the dried basis, to a tared, wide-mouth centrifuge bottle, and add hot water to obtain a total weight of the sample and water of 500.0 g. Capping the bottle, stir by mechanical means at 400 ± 50 rpm for 10–20 min until the particles are thoroughly dispersed and wetted out. Scrape down the walls of the bottle with a spatula, if necessary, to ensure that there is no undissolved material on the sides of the bottle, and continue the stirring in a cooling water bath equilibrated at a temperature below 10 for another 20–40 min. Adjust the solution weight if necessary to 500.0 g, using cold water. Centrifuge the solution, if necessary, to expel any entrapped air. If any foam is present, remove with a spatula.
for another 20–40 min. Adjust the solution weight if necessary to 500.0 g, using cold water. Centrifuge the solution, if necessary, to expel any entrapped air. If any foam is present, remove with a spatula.
Analysis:
Equip a suitable single-cylinder type rotational viscosimeter (Brookfield type LV Model, or equivalent), and determine the viscosity of this solution at 20 ± 0.1 under the operating conditions specified in the table below.
under the operating conditions specified in the table below.
| Labeled Viscositya (mPa·s) | Rotor No. |
Revolution (rpm) |
Calculation Multiplier |
|---|---|---|---|
| 600 or more and less than 1400 | 3 | 60 | 20 |
| 1400 or more and less than 3500 | 3 | 12 | 100 |
| 3500 or more and less than 9500 | 4 | 60 | 100 |
| 9500 or more and less than 99,500 | 4 | 6 | 1000 |
| 99,500 or more | 4 | 3 | 2000 |
|
a
The Labeled Viscosity is based on the manufacturer's specifications.
|
|||
Allow the spindle to rotate for 2 min before taking the measurement. Allow a rest period of 2 min between subsequent measurements. Repeat the operation twice to rotate the spindle as specified above, and average the three readings.
Acceptance criteria:
75%–140% of the viscosity stated on the label
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in well-closed containers. No storage requirements are specified.
• Labeling:
Label it to indicate its substitution type and its nominal viscosity value in millipascals per second(mPa·s).
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Robert H. Lafaver, M.S.
Scientific Liaison 1-301-816-8335 |
(EXC2010) Monographs - Excipients |
USP35–NF30 Page 3467
Pharmacopeial Forum: Volume No. 32(5) Page 1573