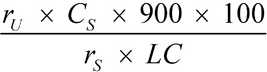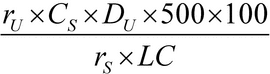Hydrocodone Bitartrate and Homatropine Methylbromide Tablets
» Hydrocodone Bitartrate and Homatropine Methylbromide Tablets contain not less than 90.0 percent and not more than 110.0 percent of the labeled amounts of hydrocodone bitartrate disesquihydrate (C18H21NO3·C4H6O6·2½ H2O) and homatropine methylbromide (C17H24BrNO3).
[note—Use of silanized autosampler vials such as dimethyldichlorosilane vials* is required for the Dissolution test, the Limit tests, and the Assay to prevent drug degradation. ]
Packaging and storage—
Preserve in tight, light-resistant containers.
Labeling—
When more than one Dissolution test is given, the labeling states the Dissolution test used only if Test 1 is not used.
Identification—
A:
Thin-Layer Chromatographic Identification Test  201
201 —
—
Solution A—
Dissolve 850 mg of bismuth subnitrate in a mixture of 10 mL of glacial acetic acid and 40 mL of water.
Solution B—
Dissolve 8 g of potassium iodide in 20 mL of water.
Stock solution:
a mixture of Solution A and Solution B (1:1).
Solvent:
a mixture of methanol and water (9:1).
Spray reagent 1—
[note—Prepare immediately before use.] Prepare a mixture of water, glacial acetic acid, and Stock solution (50:10:5).
Spray reagent 2:
hydrogen peroxide TS.
Standard solution 1—
Transfer an accurately weighed quantity of about 30 mg of USP Homatropine Methylbromide RS to a 100-mL volumetric flask, dissolve in and dilute with Solvent to volume, and mix.
Standard solution 2—
Transfer an accurately weighed quantity of about 25 mg of USP Hydrocodone Bitartrate RS to a 25-mL volumetric flask, dissolve in and dilute with Solvent to volume, and mix.
Test solution—
Transfer a portion of 20 finely powdered Tablets, equivalent to the average Tablet weight, to a centrifuge tube, add 5.0 mL of Solvent, centrifuge, and use the supernatant.
Developing solvent system:
a mixture of ethyl acetate, water, and formic acid (134:33:33).
Procedure—
Apply 50 µL of Standard solution 1, Standard solution 2, and the Test solution, and proceed as directed in the chapter. Remove the plate, and dry at 105 . Spray the plate with Spray reagent 1 and then with Spray reagent 2: the RF values for the principal spots in the chromatogram of the Test solution correspond to those of the Standard solutions.
. Spray the plate with Spray reagent 1 and then with Spray reagent 2: the RF values for the principal spots in the chromatogram of the Test solution correspond to those of the Standard solutions.
B:
The retention times of the major peaks in the chromatogram of the Assay preparation correspond to those in the chromatogram of the Standard preparation, as obtained in the Assay.
Dissolution  711
711 —
—
test 1—
Medium:
water; 900 mL, deaerated.
Apparatus 2:
50 rpm.
Time:
30 minutes.
Determine the amounts of hydrocodone bitartrate and homatropine bromide dissolved employing the following method.
Buffer solution and Mobile phase—
Proceed as directed in the Assay.
Standard solution—
Dissolve an accurately weighed quantity of USP Hydrocodone Bitartrate RS and USP Homatropine Methylbromide RS in Medium, and dilute quantitatively with Medium to obtain a solution having known concentrations of about 0.0055 mg per mL and 0.00165 mg per mL, respectively.
Test solution—
Use the solution under test passed through a suitable 0.45-µm filter.
Chromatographic system—
Proceed as directed in the Assay, using the Standard solution: the resolution, R, between homatropine methylbromide and hydrocodone bitartrate is not less than 13; and the relative standard deviation for replicate injections is not more than 3.0% for each analyte.
Procedure—
Separately inject equal volumes (about 250 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the percentage of hydrocodone bitartrate and homatropine methylbromide dissolved by the formula:
in which rU and rS are the peak areas of each drug substance in the Test solution and in the Standard solution, respectively; CS is the concentration of each drug substance, in mg per mL, in the Standard solution; 900 is the volume, in mL, of Medium; 100 is the conversion factor to percentage; and LC is the tablet label claim for each drug substance, in mg.
Tolerances—
Not less than 80% (Q) of hydrocodone bitartrate and homatropine methylbromide is dissolved in 30 minutes.
test 2—
If the product complies with this test, the labeling indicates that the product meets USP Dissolution Test 2.
Medium:
water, 500 mL.
Apparatus 2:
50 rpm.
Time:
45 minutes.
Determine the amounts of hydrocodone bitartrate and homatropine bromide dissolved employing the following method.
Mobile phase—
Prepare a filtered and degassed mixture of water and acetonitrile (3:1) that contains 1.4 g of octanesulfonic acid sodium salt and 1.0 mL of phosphoric acid per 1000 mL. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Hydrocodone bitartrate standard solution—
Transfer about 50 mg, accurately weighed, of USP Hydrocodone Bitartrate RS to a 100-mL volumetric flask, and dissolve in and dilute with Mobile phase to volume.
Homatropine methylbromide standard solution—
Transfer about 37.5 mg, accurately weighed, of USP Homatropine Methylbromide RS to a 250-mL volumetric flask, dissolve in and dilute with Mobile phase to volume.
System suitability solution—
Transfer 10.0 mL of Hydrocodone bitartrate standard solution and Homatropine methylbromide standard solution to a 500-mL volumetric flask, add 105 mL of Mobile phase. Dilute with Medium to volume.
Test solution—
Pass a portion of 20 mL of the solution under test through a suitable 0.45-µm filter, discarding the first 2 to 3 mL. Mix thoroughly 15.0 mL of the filtrate with 5.0 mL of Mobile phase.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 212-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 2.0 mL per minute. Chromatograph the System suitability solution, and record the chromatogram as directed for Procedure: the tailing factor for each drug substance is not more than 1.5; the resolution, R, between homatropine methylbromide and hydrocodone bitartrate is not less than 2.2; and the relative standard deviation for replicate injections is not more than 3.0% for homatropine methylbromide and not more than 2.0% for hydrocodone bitartrate.
)—
The liquid chromatograph is equipped with a 212-nm detector and a 3.9-mm × 30-cm column that contains packing L1. The flow rate is about 2.0 mL per minute. Chromatograph the System suitability solution, and record the chromatogram as directed for Procedure: the tailing factor for each drug substance is not more than 1.5; the resolution, R, between homatropine methylbromide and hydrocodone bitartrate is not less than 2.2; and the relative standard deviation for replicate injections is not more than 3.0% for homatropine methylbromide and not more than 2.0% for hydrocodone bitartrate.
Procedure—
Separately inject equal volumes (about 200 µL) of the appropriate Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentage of each drug substance dissolved by the formula:
in which rU and rS are the peak responses of each drug substance in the Test solution and in the correspondent Standard solution, respectively; CS is the concentration of each drug substance, in mg per mL, in the correspondent Standard solution; DU is the dilution factor of the Test solution; 500 is the volume, in mL, of Medium; 100 is the conversion factor to percentage; and LC is the tablet label claim for each drug substance, in mg.
Tolerances—
Not less than 75% (Q) of hydrocodone bitartrate and homatropine methylbromide is dissolved in 45 minutes.
Uniformity of dosage units  905
905 :
meet the requirements.
:
meet the requirements.
Limit of dihydrocodeine bitartrate, hydrocodone diol, and related substances—
Ion-pair solution—
Prepare 0.005 M sodium 1-octanesulfonate, and adjust with glacial acetic acid to a pH of 2.5 ± 0.1.
Mobile phase—
Prepare a filtered and degassed mixture of Ion-pair solution and methanol (6:4). Add 0.5 mL of triethylamine per L. Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
System suitability solution—
Dissolve about 2 mg each of hydrocodone diol and USP Dihydrocodeine Bitartrate RS in 35 mL of Mobile phase in a 100-mL volumetric flask, and dilute with Mobile phase to volume. Dilute this solution quantitatively, and stepwise if necessary, with Mobile phase to obtain a solution having a known concentration of each of about 0.1 µg per mL.
Standard solution—
Use the Standard preparation, prepared as directed in the Assay.
Test solution—
Use the Assay preparation.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 280-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L7. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.67 for hydrocodone diol, 0.75 for dihydrocodeine bitartrate, and 1.0 for hydrocodone bitartrate; the resolution, R, between hydrocodone diol and dihydrocodeine bitartrate is not less than 2.0; and the relative standard deviation for replicate injections of each of these compounds is not more than 5.0%.
)—
The liquid chromatograph is equipped with a 280-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L7. The flow rate is about 1.5 mL per minute. Chromatograph the System suitability solution, and record the peak responses as directed for Procedure: the relative retention times are about 0.67 for hydrocodone diol, 0.75 for dihydrocodeine bitartrate, and 1.0 for hydrocodone bitartrate; the resolution, R, between hydrocodone diol and dihydrocodeine bitartrate is not less than 2.0; and the relative standard deviation for replicate injections of each of these compounds is not more than 5.0%.
Procedure—
Separately inject equal volumes (about 200 µL) of the Standard solution and Test solution into the chromatograph, record the chromatograms, and measure the peak areas. Calculate the percentages of hydrocodone diol and dihydrocodeine bitartrate in the portion of Tablets taken by the formula:
100(rD / rS)
in which rD is the individual peak response of either hydrocodone diol or dihydrocodeine in the chromatogram obtained from the Test solution; and rS is the peak response of hydrocodone bitartrate in the chromatogram obtained from the Standard solution: not more than 0.5% of hydrocodone diol is found, and not more than 1.0% of dihydrocodeine bitartrate is found. Calculate the percentage of each other related substance in the portion of Tablets taken by the formula:
100(ri / rs)
in which ri is the peak response for any individual related substance with a retention time greater than 5 minutes; and rs is the sum of the responses of all the peaks: not more than 0.5% of any individual related substance is found. The sum of all impurities is not more than 1.5%.
Limit of homatropine hydrobromide and related substances—
Buffer solution—
Prepare a solution of 0.005 M dibasic potassium phosphate, and adjust with phosphoric acid to a pH of 6.4 ± 0.1.
Mobile phase—
Prepare a filtered and degassed mixture of Buffer solution and acetonitrile (17:3). Make adjustments if necessary (see System Suitability under Chromatography  621
621 ).
).
Standard solution—
Dissolve an accurately weighed quantity of homatropine hydrobromide in Mobile phase, and dilute quantitatively with Mobile phase to obtain a solution having a known concentration of about 0.6 µg per mL.
Test solution—
Use the Assay preparation.
Chromatographic system (see Chromatography  621
621 )—
The liquid chromatograph is equipped with a 210-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L7. The flow rate is about 1.5 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 5.0%.
)—
The liquid chromatograph is equipped with a 210-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L7. The flow rate is about 1.5 mL per minute. Chromatograph the Standard solution, and record the peak responses as directed for Procedure: the relative standard deviation for replicate injections is not more than 5.0%.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard solution and the Test solution into the chromatograph, record the chromatograms, and measure the peak responses. Calculate the percentage of homatropine hydrobromide in the portion of Tablets taken by the formula:
100(rH / rS)
in which rH is the individual peak response of homatropine hydrobromide in the chromatogram obtained from the Test solution; and rS is the peak response for homatropine methylbromide in the chromatogram obtained from the Standard solution: not more than 0.5% of homatropine hydrobromide is found. Calculate the percentage of each other related substance in the portion of Tablets taken by the formula:
100(ri / rs)
in which ri is the peak response for any individual related substance with a relative retention time less than 0.44 in relation to the retention time of hydrocodone bitartrate; and rs is the sum of the responses of all the peaks: not more than 0.5% of any individual related substance is found. The sum of all impurities is not more than 1.5%.
Limit of tropine—
Adsorbent:
0.25-mm layer of chromatographic silica gel.
Diluent:
diethyl ether.
Test solution—
Finely powder 25 Tablets, and add to a centrifuge tube. Pipet 5.0 mL of diethyl ether into the centrifuge tube, mix on a vortex mixer for 5 minutes, centrifuge, and use the supernatant.
Standard stock solution—
Dissolve an accurately weighed quantity of tropine in Diluent, and dilute quantitatively with Diluent to obtain a solution having a known concentration of about 150 µg per mL.
Stock solutions—
Transfer 5.0 mL of the Standard stock solution to a 10-mL volumetric flask, and dilute with Diluent to volume quantitatively, and stepwise if necessary, to obtain Standard solutions A, B, C, and D having known concentrations of about 75 µg per mL, 37.5 µg per mL, 18.75 µg per mL, and 9.38 µg per mL, respectively.
Spray reagent—
Dissolve 300 mg of platinic acid in 3 mL of diluted hydrochloric acid, add 97 mL of water and 100 mL of 6% potassium iodide in water, and mix.
Developing solvent system:
a mixture of alcohol and ammonium hydroxide (400:100).
Procedure—
Apply equal volumes (about 500 µL) of the Standard stock solution, Standard solutions A, B, C, and D, and the Test solution to a thin-layer chromatographic plate (see Chromatography  621
621 ), and proceed as directed in the chapter. After the plate has dried, position it in a chamber saturated with iodine vapor for about 30 minutes, then place it in a hood to allow the iodine to sublime from the plate, and spray the plate with Spray reagent until spots appear. Any spot from the Test solution occurring at an RF value corresponding to tropine is not greater in size or intensity than the corresponding spot obtained from Standard solution B (0.5%): not more than 0.5% of tropine is found.
), and proceed as directed in the chapter. After the plate has dried, position it in a chamber saturated with iodine vapor for about 30 minutes, then place it in a hood to allow the iodine to sublime from the plate, and spray the plate with Spray reagent until spots appear. Any spot from the Test solution occurring at an RF value corresponding to tropine is not greater in size or intensity than the corresponding spot obtained from Standard solution B (0.5%): not more than 0.5% of tropine is found.
Assay—
Buffer solution—
Prepare a solution of 0.005 M dibasic potassium phosphate, and adjust with phosphoric acid to a pH of 6.4 ± 0.01.
Mobile phase—
Prepare a filtered and degassed mixture of Buffer solution and acetonitrile (17:3).
Standard preparation—
Dissolve an accurately weighed quantity of USP Hydrocodone Bitartrate RS and USP Homatropine Methylbromide RS in Mobile phase, and dilute quantitatively with Mobile phase to obtain a solution having known concentrations of about 0.2 mg per mL and 0.06 mg per mL, respectively.
Assay preparation—
Weigh and finely powder not fewer than 20 Tablets. Transfer an accurately weighed portion of the powder, equivalent to about 5 mg of hydrocodone bitartrate and about 1.5 mg of homatropine methylbromide, to a 25-mL volumetric flask. Pipet 15 mL of the Mobile phase into the volumetric flask, sonicate for 15 minutes, and then shake with a wrist-action shaker for 15 additional minutes. Pipet an additional 10 mL of Mobile phase into the volumetric flask, and mix. Pass the solution through a filter having a 0.45-µm porosity prior to injection into the chromatograph.
Chromatographic system—
The liquid chromatograph is equipped with a 230-nm detector and a 4.6-mm × 25-cm column that contains 5-µm packing L7. The flow rate is about 1.5 mL per minute. Chromatograph the Standard preparation, and record the peak responses as directed for Procedure: the relative retention times are about 0.44 for homatropine methylbromide and 1.0 for hydrocodone bitatrate; the resolution, R, between hydrocodone bitartrate and homatropine methylbromide is not less than 2.5; and the relative standard deviation for replicate injections is not more than 3.0% for each analyte.
Procedure—
Separately inject equal volumes (about 10 µL) of the Standard preparation and the Assay preparation into the chromatograph, record the chromatograms, and measure the areas for the major peaks. Calculate the amount, in mg, of homatropine methylbromide (C17H24BrNO3) in the portion of Tablets taken by the formula:
(LCS / CU)(rU / rS)
in which L is the labeled quantity, in mg, of homatropine methylbromide in each Tablet; CS is the concentration, in mg per mL, of USP Homatropine Methylbromide RS in the Standard preparation; CU is the concentration, in mg per mL, of homatropine methylbromide in the Assay preparation, based on the labeled amount per Tablet and the extent of dilution; and rU and rS are the homatropine methylbromide peak responses obtained from the Assay preparation and the Standard preparation, respectively. Calculate the amount, in mg, of hydrocodone bitartrate disesquihydrate (C18H21NO3·C4H6O6·2½ H2O) in the portion of Tablets taken by the formula:
(494.50/449.46)(LCS / CU)(rU / rS)
in which 494.50 and 449.46 are the molecular weights of hydrocodone bitartrate disesquihydrate and anhydrous hydrocodone bitartrate, respectively; L is the labeled amount, in mg, of hydrocodone bitartrate disesquihydrate in each Tablet; CS is the concentration, in mg per mL, of USP Hydrocodone Bitartrate RS in the Standard preparation; CU is the concentration, in mg per mL, of hydrocodone bitartrate disesquihydrate in the Assay preparation, based on the labeled amount per Tablet and the extent of dilution; and rU and rS are the peak responses obtained from the Assay preparation and the Standard preparation, respectively.
*
A suitable grade is available from Analytical Research and Testing, Somerville, NJ; Fax: 908-725-8848.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Clydewyn M. Anthony, Ph.D.
Senior Scientific Liaison 1-301-816-8139 |
(SM22010) Monographs - Small Molecules 2 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
|
| Margareth R.C. Marques, Ph.D.
Senior Scientific Liaison 1-301-816-8106 |
(GCDF2010) General Chapters - Dosage Forms |
USP35–NF30 Page 3427
Pharmacopeial Forum: Volume No. 33(4) Page 654