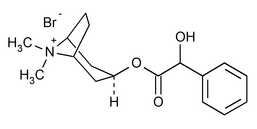
Homatropine Methylbromide
C17H24BrNO3 370.28
8-Azoniabicyclo[3.2.1]octane, 3-(hydroxyphenylacetyl)oxy-8,8-dimethyl-, bromide, endo-(±)-;
3 -Hydroxy-8-methyl-1
-Hydroxy-8-methyl-1 H,5
H,5 H-tropanium bromide mandelate;
H-tropanium bromide mandelate;
(1R,3s,5S)-3-[[(2RS)-2-Hydroxy-2-phenylacetyl]oxy]-8,8-dimethyl-8-azoniabicyclo[3.2.1]octane bromide

 [80-49-9].
[80-49-9].
(hoe mat' roe peen meth'' il broe' mide).
C17H24BrNO3 370.28
8-Azoniabicyclo[3.2.1]octane, 3-(hydroxyphenylacetyl)oxy-8,8-dimethyl-, bromide, endo-(±)-;
3
(1R,3s,5S)-3-[[(2RS)-2-Hydroxy-2-phenylacetyl]oxy]-8,8-dimethyl-8-azoniabicyclo[3.2.1]octane bromide
DEFINITION
Homatropine Methylbromide contains NLT 98.0% and NMT 102.0% of C17H24BrNO3, calculated on the dried basis.
IDENTIFICATION
• A. Infrared Absorption  197K
197K
[Note—If differences are observed, dissolve the specimen and the Reference Standard separately in methanol, and recrystallize by adding dioxane to each solution. ]
• B. Identification Tests—General, Bromide  191
191
Sample solution:
50 mg/mL in water
Acceptance criteria:
Meets the requirements
ASSAY
Procedure
Solution A:
3.4 g/L of monobasic potassium phosphate and 5 g/L of 1-pentanesulfonic acid sodium salt in water. Adjust with a 330-g/L solution of phosphoric acid to a pH of 3.0.
Solution B:
Acetonitrile and Solution A (3:2)
Diluent:
Acetonitrile and Solution A (9:41)
Mobile phase:
See the gradient table below.
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 70 | 30 |
| 2 | 70 | 30 |
| 15 | 30 | 70 |
| 15.1 | 70 | 30 |
| 20 | 70 | 30 |
System suitability solution:
0.01 mg/mL each of USP Homatropine Methylbromide RS and USP Homatropine Hydrobromide RS in Diluent
Standard solution:
2.0 mg/mL of USP Homatropine Methylbromide RS in Diluent
Sample solution:
2.0 mg/mL of Homatropine Methylbromide in Diluent
Chromatographic system
Mode:
LC
Detector:
UV 210 nm
Column:
4.6-mm × 15-cm; 3-µm packing L1
Flow rate:
1.4 mL/min
Injection size:
5 µL
System suitability
Samples:
System suitability solution and Standard solution
[Note—The relative retention times for homatropine methylbromide and homatropine hydrobromide are 1.0 and 1.14, respectively. ]
Suitability requirements
Resolution:
NLT 2.5 between homatropine methylbromide and homatropine hydrobromide, System suitability solution
Tailing factor:
NMT 1.5 for homatropine methylbromide peak, System suitability solution
Relative standard deviation:
NMT 1%, Standard solution
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of C17H24BrNO3 in the portion of Homatropine Methylbromide taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of Homatropine Methylbromide from the Sample solution |
| rS | = | = peak response of homatropine methylbromide from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
Acceptance criteria:
98.0%–102.0% on the dried basis
IMPURITIES
Inorganic Impurities
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
Organic Impurities
• Procedure
Solution A, Solution B, Diluent, Mobile phase, System suitability solution, and Sample solution:
Proceed as directed in the Assay.
Standard solution:
0.01 mg/mL of USP Homatropine Methylbromide RS in Diluent
Chromatographic system:
Proceed as directed in the Assay, except for injection size.
Injection size:
10 µL
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 2.5 between homatropine methylbromide and homatropine hydrobromide
Tailing factor:
NMT 1.5 for the homatropine methylbromide peak
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of any individual impurity in the portion of Homatropine Methylbromide taken:
Result = (rU/rS) × (CS/CU) ×100
| rU | = | = peak response of each individual impurity from the Sample solution |
| rS | = | = peak response of homatropine methylbromide from the Standard solution |
| CS | = | = concentration of the Standard solution (mg/mL) |
| CU | = | = concentration of the Sample solution (mg/mL) |
[Note—Reporting level for impurities is 0.05%. ]
Acceptance criteria
Individual impurities:
See Impurity Table 1.
Total impurities:
NMT 1.0%
[Note—Disregard the peak due to the bromide ion that elutes close to the solvent peak at about 1 min. ]
Impurity Table 1
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Methyldehydrohomatropine bromidea | 0.94 | 0.5 |
| Homatropine methylbromide | 1.0 | — |
| Homatropine hydrobromide | 1.1 | 0.5 |
| Any other individual impurity | — | 0.1 |
|
a
(1R,3s,5S)-3-[[(2RS)-2-Hydroxy-2-phenylacetyl]oxy]-8,8-dimethyl-8-azoniabicyclo[3.2.1]oct-6-ene.
|
||
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample at 105
:
Dry a sample at 105 to constant weight: it loses NMT 0.5% of its weight.
to constant weight: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in tight, light-resistant containers. Store at room temperature.
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Elena Gonikberg, Ph.D.
Principal Scientific Liaison 1-301-816-8251 |
(SM32010) Monographs - Small Molecules 3 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3415
Pharmacopeial Forum: Volume No. 36(5) Page 1188