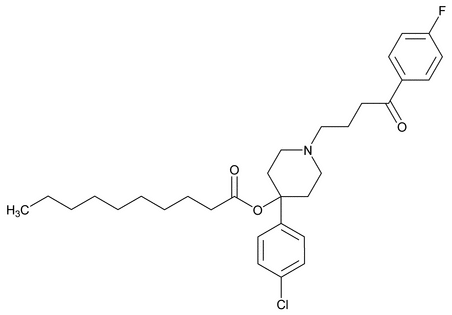
Haloperidol Decanoate
C31H41ClFNO3 530.11
Decanoic acid, 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]-4-piperidinyl ester;
Decanoic acid, ester with 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4¢-fluorobutyrophenone

 [74050-97-8].
[74050-97-8].
(hal'' oh per' i dol dek'' a noe' ate).
C31H41ClFNO3 530.11
Decanoic acid, 4-(4-chlorophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]-4-piperidinyl ester;
Decanoic acid, ester with 4-[4-(p-chlorophenyl)-4-hydroxypiperidino]-4¢-fluorobutyrophenone
DEFINITION
Haloperidol Decanoate contains NLT 97.0% and NMT 103.0% of C31H41ClFNO3, calculated on the dried basis.
IDENTIFICATION
• B.
The retention time of the major peak in the chromatogram of the Sample solution corresponds to that of the Standard solution, as obtained in the Assay.
• C. Identification Tests—General, Chloride  191
191
Sample solution:
Mix 0.1 g of the sample with 0.5 g of anhydrous sodium carbonate in a porcelain crucible. Heat over open flame for 10 min. Allow to cool. Dissolve the residue in 5 mL of dilute nitric acid, and filter. Dilute 1 mL of the filtrate with 1 mL of water.
Acceptance criteria:
Meets the requirements of the silver nitrate precipitate test
ASSAY
• Procedure
Solution A:
27 g/L of tetrabutylammonium hydrogen sulfate in water
Solution B:
Acetonitrile
Mobile phase:
See Table 1.
Table 1
| Time (min) |
Solution A (%) |
Solution B (%) |
|---|---|---|
| 0 | 80 | 20 |
| 30 | 40 | 60 |
| 35 | 40 | 60 |
| 40 | 80 | 20 |
| 45 | 80 | 20 |
Standard solution:
0.2 mg/mL of USP Haloperidol Decanoate RS in methanol
Sample solution:
0.2 mg/mL of Haloperidol Decanoate in methanol
Chromatographic system
Mode:
LC
Detector:
UV 230 nm
Column:
4-mm × 10-cm; 3-µm packing L1
Flow rate:
1.5 mL/min
Injection size:
10 µL
System suitability
Sample:
Standard solution
Suitability requirements
Tailing factor:
NMT 1.5
Relative standard deviation:
NMT 2.0%
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of Haloperidol Decanoate (C31H41ClFNO3) in the portion of sample taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response from the Sample solution |
| rS | = | = peak response from the Standard solution |
| CS | = | = concentration of USP Haloperidol Decanoate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Haloperidol Decanoate in the Sample solution (mg/mL) |
Acceptance criteria:
97.0%–103.0% on the dried basis
IMPURITIES
• Residue on Ignition  281
281 :
NMT 0.1%
:
NMT 0.1%
• Heavy Metals, Method II  231
231 :
NMT 20 ppm
:
NMT 20 ppm
• Organic Impurities
Solution A, Solution B, and Mobile phase:
Proceed as directed in the Assay.
System suitability solution:
0.05 mg/mL each of USP Haloperidol Decanoate RS and USP Bromperidol Decanoate RS in methanol
Standard stock solution:
0.2 mg/mL of USP Haloperidol Decanoate RS in methanol
Standard solution:
0.05 mg/mL of USP Haloperidol Decanoate RS in methanol, from Standard stock solution
Sample solution:
10 mg/mL of Haloperidol Decanoate in methanol
Chromatographic system:
Proceed as directed in the Assay.
System suitability
Sample:
System suitability solution
Suitability requirements
Resolution:
NLT 1.5 between haloperidol decanoate and bromperidol decanoate
Analysis
Samples:
Standard solution and Sample solution
Calculate the percentage of each impurity in the portion of Haloperidol Decanoate taken:
Result = (rU/rS) × (CS/CU) × 100
| rU | = | = peak response of each impurity from the Sample solution |
| rS | = | = peak response of haloperidol decanoate from the Standard solution |
| CS | = | = concentration of USP Haloperidol Decanoate RS in the Standard solution (mg/mL) |
| CU | = | = concentration of Haloperidol Decanoate in the Sample solution (mg/mL) |
Acceptance criteria:
See Table 2.
Table 2
| Name | Relative Retention Time |
Acceptance Criteria, NMT (%) |
|---|---|---|
| Haloperidola | 0.09 | 0.50 |
| Haloperidol octanoateb | 0.6 | 0.50 |
| Haloperidol nonanoatec | 0.79 | 0.50 |
| Haloperidol decanoate-dechloro analogd | 0.89 | 0.50 |
| 2-Fluorohaloperidol decanoatee | 0.97 | 0.50 |
| Haloperidol decanoate | 1.0 | — |
| Bromperidol decanoatef | 1.05 | — |
| Haloperidol undecanoateg | 1.10 | 0.50 |
| Haloperidol decanoate-3-ethyl analogh | 1.13 | 0.50 |
| Haloperidol decanoate-4- piperidinol analogi |
1.17 | 0.50 |
| Haloperidol dodecanoatej | 1.21 | 0.50 |
| Haloperidol decanoate-3-chlorobiphenyl analogk | 1.22 | 0.50 |
| Haloperidol decanoate-4-chlorobiphenyl analogl | 1.24 | 0.50 |
| Any other individual, unspecified impurity | — | 0.10 |
| Total impurities | — | 1.0 |
|
a
4-[4-(4-Chlorophenyl)-4-hydroxypiperidino]-4-fluorobutyrophenone.
b
4-(4-Chlorophenyl)-1-(4-(4-fluorophenyl)-4-oxobutyl)piperidin-4-yl octanoate.
c
4-(4-Chlorophenyl)-1-(4-(4-fluorophenyl)-4-oxobutyl)piperidin-4-yl nonanoate.
d
1-[4-(4-Fluorophenyl)-4-oxobutyl]-4-phenylpiperidin-4-yl decanoate.
e
4-(4-Chlorophenyl)-1-(4-(2-fluorophenyl)-4-oxobutyl)piperidin-4-yl decanoate.
f
Used only for system suitability.
g
4-(4-Chlorophenyl)-1-(4-(4-fluorophenyl)-4-oxobutyl)piperidin-4-yl undecanoate.
h
4-(4-Chlorophenyl)-1-[4-(3-ethyl-4-fluorophenyl)-4-oxobutyl]piperidin-4-yl decanoate.
i
4-(4-Chlorophenyl)-1-(4-{4-[4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl]phenyl}-4-oxobutyl)piperidin-4-yl decanoate.
j
4-(4-Chlorophenyl)-1-(4-(4-fluorophenyl)-4-oxobutyl)piperidin-4-yl dodecanoate.
k
4-(4¢-Chlorobiphenyl-4-yl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl decanoate.
l
4-(3¢-Chlorobiphenyl-4-yl)-1-[4-(4-fluorophenyl)-4-oxobutyl]piperidin-4-yl decanoate.
|
||
SPECIFIC TESTS
• Loss on Drying  731
731 :
Dry a sample in a vacuum at 30
:
Dry a sample in a vacuum at 30 under phosphorous pentoxide desiccant: it loses NMT 0.5% of its weight.
under phosphorous pentoxide desiccant: it loses NMT 0.5% of its weight.
ADDITIONAL REQUIREMENTS
• Packaging and Storage:
Preserve in light-resistant, tight containers. Store at room temperature.
• USP Reference Standards  11
11
USP Bromperidol Decanoate RS
Decanoic acid, 4-(4-bromophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]-4-piperidinyl ester.
C31H41BrFNO3 574.56
Decanoic acid, 4-(4-bromophenyl)-1-[4-(4-fluorophenyl)-4-oxobutyl]-4-piperidinyl ester.
C31H41BrFNO3 574.56
USP Haloperidol Decanoate RS
Auxiliary Information—
Please check for your question in the FAQs before contacting USP.
| Topic/Question | Contact | Expert Committee |
|---|---|---|
| Monograph | Ravi Ravichandran, Ph.D.
Principal Scientific Liaison 1-301-816-8330 |
(SM42010) Monographs - Small Molecules 4 |
| Reference Standards | RS Technical Services 1-301-816-8129 rstech@usp.org |
USP35–NF30 Page 3399
Pharmacopeial Forum: Volume No. 34(3) Page 614